Mutation in Osteoactivin Promotes Receptor Activator of NFκB Ligand (RANKL)-mediated Osteoclast Differentiation and Survival but Inhibits Osteoclast Function
- PMID: 25837253
- PMCID: PMC4536424
- DOI: 10.1074/jbc.M114.624270
Mutation in Osteoactivin Promotes Receptor Activator of NFκB Ligand (RANKL)-mediated Osteoclast Differentiation and Survival but Inhibits Osteoclast Function
Abstract
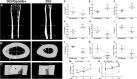
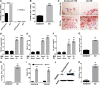
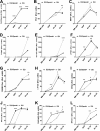
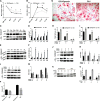
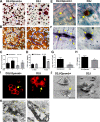
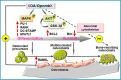
We previously reported on the importance of osteoactivin (OA/Gpnmb) in osteogenesis. In this study, we examined the role of OA in osteoclastogenesis, using mice with a nonsense mutation in the Gpnmb gene (D2J) and wild-type controls (D2J/Gpnmb(+)). In these D2J mice, micro-computed tomography and histomorphometric analyses revealed increased cortical thickness, whereas total porosity and eroded surface were significantly reduced in D2J mice compared with wild-type controls, and these results were corroborated by lower serum levels of CTX-1. Contrary to these observations and counterintuitively, temporal gene expression analyses supported up-regulated osteoclastogenesis in D2J mice and increased osteoclast differentiation rates ex vivo, marked by increased number and size. The finding that MAPK was activated in early differentiating and mature D2J osteoclasts and that survival of D2J osteoclasts was enhanced and mediated by activation of the AKT-GSK3β pathway supports this observation. Furthermore, this was abrogated by the addition of recombinant OA to cultures, which restored osteoclastogenesis to wild-type levels. Moreover, mix and match co-cultures demonstrated an induction of osteoclastogenesis in D2J osteoblasts co-cultured with osteoclasts of D2J or wild-type. Last, in functional osteo-assays, we show that bone resorption activity of D2J osteoclasts is dramatically reduced, and these osteoclasts present an abnormal ruffled border over the bone surface. Collectively, these data support a model whereby OA/Gpnmb acts as a negative regulator of osteoclast differentiation and survival but not function by inhibiting the ERK/AKT signaling pathways.
Keywords: Akt; MAP kinases (MAPKs); bone; bone marrow; osteoactivin; osteoclast; osteopetrosis.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Lassus J., Salo J., Jiranek W. A., Santavirta S., Nevalainen J., Matucci-Cerinic M., Horak P., Konttinen Y. (1998) Macrophage activation results in bone resorption. Clin. Orthop. Relat. Res. 7–15 - PubMed
-
- Anderson J. M. (2000) Multinucleated giant cells. Curr. Opin. Hematol. 7, 40–47 - PubMed
-
- Kukita T., Kukita A. (1996) Osteoclast differentiation antigen. Histol. Histopathol. 11, 821–830 - PubMed
-
- Pogoda P., Priemel M., Rueger J. M., Amling M. (2005) Bone remodeling: new aspects of a key process that controls skeletal maintenance and repair. Osteoporos. Int. 16, S18–S24 - PubMed
-
- Lemaire V., Tobin F. L., Greller L. D., Cho C. R., Suva L. J. (2004) Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J. Theor. Biol. 229, 293–309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

