Computational tools for epitope vaccine design and evaluation
- PMID: 25837467
- PMCID: PMC4456225
- DOI: 10.1016/j.coviro.2015.03.013
Computational tools for epitope vaccine design and evaluation
Abstract
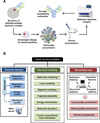
Rational approaches will be required to develop universal vaccines for viral pathogens such as human immunodeficiency virus, hepatitis C virus, and influenza, for which empirical approaches have failed. The main objective of a rational vaccine strategy is to design novel immunogens that are capable of inducing long-term protective immunity. In practice, this requires structure-based engineering of the target neutralizing epitopes and a quantitative readout of vaccine-induced immune responses. Therefore, computational tools that can facilitate these two areas have played increasingly important roles in rational vaccine design in recent years. Here we review the computational techniques developed for protein structure prediction and antibody repertoire analysis, and demonstrate how they can be applied to the design and evaluation of epitope vaccines.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Current progress of immunoinformatics approach harnessed for cellular- and antibody-dependent vaccine design.Pathog Glob Health. 2018 May;112(3):123-131. doi: 10.1080/20477724.2018.1446773. Epub 2018 Mar 12. Pathog Glob Health. 2018. PMID: 29528265 Free PMC article.
-
iVAX: An integrated toolkit for the selection and optimization of antigens and the design of epitope-driven vaccines.Hum Vaccin Immunother. 2015;11(9):2312-21. doi: 10.1080/21645515.2015.1061159. Hum Vaccin Immunother. 2015. PMID: 26155959 Free PMC article.
-
Immunoinformatics: In Silico Approaches and Computational Design of a Multi-epitope, Immunogenic Protein.Int Rev Immunol. 2019;38(6):307-322. doi: 10.1080/08830185.2019.1657426. Epub 2019 Sep 3. Int Rev Immunol. 2019. PMID: 31478759
-
Advances in structure-based vaccine design.Curr Opin Virol. 2013 Jun;3(3):322-31. doi: 10.1016/j.coviro.2013.05.010. Epub 2013 Jun 25. Curr Opin Virol. 2013. PMID: 23806515 Free PMC article. Review.
-
Structural and Computational Biology in the Design of Immunogenic Vaccine Antigens.J Immunol Res. 2015;2015:156241. doi: 10.1155/2015/156241. Epub 2015 Oct 7. J Immunol Res. 2015. PMID: 26526043 Free PMC article. Review.
Cited by
-
Emerging Concepts and Technologies in Vaccine Development.Front Immunol. 2020 Sep 30;11:583077. doi: 10.3389/fimmu.2020.583077. eCollection 2020. Front Immunol. 2020. PMID: 33101309 Free PMC article. Review.
-
Strategies Using Bio-Layer Interferometry Biosensor Technology for Vaccine Research and Development.Biosensors (Basel). 2017 Oct 31;7(4):49. doi: 10.3390/bios7040049. Biosensors (Basel). 2017. PMID: 29088096 Free PMC article. Review.
-
Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01-Class Precursor Antibodies.Immunity. 2018 Aug 21;49(2):301-311.e5. doi: 10.1016/j.immuni.2018.07.005. Epub 2018 Jul 31. Immunity. 2018. PMID: 30076101 Free PMC article.
-
Variability in Humoral Immunity to Measles Vaccine: New Developments.Trends Mol Med. 2015 Dec;21(12):789-801. doi: 10.1016/j.molmed.2015.10.005. Epub 2015 Nov 18. Trends Mol Med. 2015. PMID: 26602762 Free PMC article. Review.
-
Systems Biology Approaches for Therapeutics Development Against COVID-19.Front Cell Infect Microbiol. 2020 Oct 28;10:560240. doi: 10.3389/fcimb.2020.560240. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194800 Free PMC article. Review.
References
-
-
Nabel GJ. Designing Tomorrow's Vaccines. New Engl J Med. 2013;368:551–560. • This review covered a wide range of topics in vaccine research, including past successes and current challenges, new conceptual and technological advances, clinical translation and implementation, and future vaccine development.
-
-
-
Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13:693–701. • The authors discussed the role of broadly neutralizing antibodies in structural analysis of antibody-epitope interactions and rational design of immunogens to target the intermediate antibodies during B-cell development.
-
-
-
Walker LM, Burton DR. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr Opin Immunol. 2010;22:358–366. •• This was one of the earliest articles describing the concept and major components of an antibody-based rational vaccine design approach. The importance of broadly neutralizing antibody identification was highlighted in the review.
-
-
-
Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–433. •• The authors extended the rational vaccine approach of Walker et al. by including the intermediate antibodies derived from a neutralizing B-cell lineage as templates to design a series of immunogens to guide the B-cell development during the vaccination.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

