Escherichia coli morphological changes and lipid A removal induced by reduced pressure nitrogen afterglow exposure
- PMID: 25837580
- PMCID: PMC4383372
- DOI: 10.1371/journal.pone.0116083
Escherichia coli morphological changes and lipid A removal induced by reduced pressure nitrogen afterglow exposure
Abstract
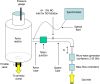
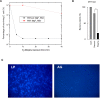
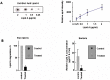
Lipid A is a major hydrophobic component of lipopolysaccharides (endotoxin) present in the membrane of most Gram-negative bacteria, and the major responsible for the bioactivity and toxicity of the endotoxin. Previous studies have demonstrated that the late afterglow region of flowing post-discharges at reduced pressure (1-20 Torr) can be used for the sterilization of surfaces and of the reusable medical instrumentation. In the present paper, we show that the antibacterial activity of a pure nitrogen afterglow can essentially be attributed to the large concentrations of nitrogen atoms present in the treatment area and not to the UV radiation of the afterglow. In parallel, the time variation of the inactivation efficiency quantified by the log reduction of the initial Escherichia coli (E. coli) population is correlated with morphologic changes observed on the bacteria by scanning electron microscopy (SEM) for increasing afterglow exposure times. The effect of the afterglow exposure is also studied on pure lipid A and on lipid A extracted from exposed E. coli bacteria. We report that more than 60% of lipid A (pure or bacteria-extracted) are lost with the used operating conditions (nitrogen flow QN2 = 1 standard liter per minute (slpm), pressure p = 5 Torr, microwave injected power PMW = 200 W, exposure time: 40 minutes). The afterglow exposure also results in a reduction of the lipid A proinflammatory activity, assessed by the net decrease of the redox-sensitive NFκB transcription factor nuclear translocation in murine aortic endothelial cells stimulated with control vs afterglow-treated (pure and extracted) lipid A. Altogether these results point out the ability of reduced pressure nitrogen afterglows to neutralize the cytotoxic components in Gram-negative bacteria.
Conflict of interest statement
Figures


Similar articles
-
E. coli, P. aeruginosa, and B. cereus bacteria sterilization using afterglow of non-thermal plasma at atmospheric pressure.Appl Biochem Biotechnol. 2010 Apr;160(7):1978-84. doi: 10.1007/s12010-009-8817-3. Epub 2009 Nov 1. Appl Biochem Biotechnol. 2010. PMID: 19882114
-
Bacterial inactivation in open air by the afterglow plume emitted from a grounded hollow slot electrode.Environ Sci Technol. 2005 Jan 1;39(1):339-44. Environ Sci Technol. 2005. PMID: 15667115
-
Visible light photocatalytic antibacterial activity of Ni-doped and N-doped TiO2 on Staphylococcus aureus and Escherichia coli bacteria.Environ Sci Pollut Res Int. 2016 Mar;23(5):4111-9. doi: 10.1007/s11356-015-4775-1. Epub 2015 Jun 2. Environ Sci Pollut Res Int. 2016. PMID: 26028352
-
Lipid A: target for antibacterial drugs.Science. 1996 Nov 8;274(5289):939-40. doi: 10.1126/science.274.5289.939. Science. 1996. PMID: 8966574 Review. No abstract available.
-
Lipopolysaccharide: Biosynthetic pathway and structure modification.Prog Lipid Res. 2010 Apr;49(2):97-107. doi: 10.1016/j.plipres.2009.06.002. Epub 2009 Oct 6. Prog Lipid Res. 2010. PMID: 19815028 Review.
Cited by
-
Involvement of multiple stressors induced by non-thermal plasma-charged aerosols during inactivation of airborne bacteria.PLoS One. 2017 Feb 6;12(2):e0171434. doi: 10.1371/journal.pone.0171434. eCollection 2017. PLoS One. 2017. PMID: 28166240 Free PMC article.
-
Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure.PLoS One. 2017 Mar 30;12(3):e0173618. doi: 10.1371/journal.pone.0173618. eCollection 2017. PLoS One. 2017. PMID: 28358809 Free PMC article.
References
-
- Vasselle A. Prévenir les infections nosocomiales: une exigence de qualité des soins hospitaliers. I.A.1. Un phénomène polymorphe aux causes multiples (in french). OPEPS report n°421. 2006. Available: http://www.senat.fr/rap/r05-421/r05-4211.html.
-
- Vasselle A. Prévenir les infections nosocomiales: une exigence de qualité des soins hospitaliers. I.A.2. Des conséquence lourdes pour le patient comme pour la société (in french). OPEPS report n°421. 2006. Available: http://www.senat.fr/rap/r05-421/r05-4213.html.
-
- Sifuentes-Osornio J, Guerrero-Almeida MC, Ponce de Leon-Garduno LA, Guerrero-Almeida ML. Tendencia de las bacteremias y factores de riesgo de muerte en un hospital de tercer nivel de la Ciudad de México. 1981 a 1992 (in spanish). Gac. Med. Mex. 2001;137: 191–202. - PubMed
-
- DesCôteaux JG, Poulin EC, Julien M, Guidoin R. Residual organic debris on processed surgical instruments. AORN J. 1995;62:23–30. - PubMed
-
- Alfa MJ, Degagne P, Olson N. Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning. Am. J. Infect. Control. 1999;27:392–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

