A TatABC-type Tat translocase is required for unimpaired aerobic growth of Corynebacterium glutamicum ATCC13032
- PMID: 25837592
- PMCID: PMC4383559
- DOI: 10.1371/journal.pone.0123413
A TatABC-type Tat translocase is required for unimpaired aerobic growth of Corynebacterium glutamicum ATCC13032
Abstract
The twin-arginine translocation (Tat) system transports folded proteins across the cytoplasmic membrane of bacteria and the thylakoid membrane of plant chloroplasts. Escherichia coli and other Gram-negative bacteria possess a TatABC-type Tat translocase in which each of the three inner membrane proteins TatA, TatB, and TatC performs a mechanistically distinct function. In contrast, low-GC Gram-positive bacteria, such as Bacillus subtilis, use a TatAC-type minimal Tat translocase in which the TatB function is carried out by a bifunctional TatA. In high-GC Gram-positive Actinobacteria, such as Mycobacterium tuberculosis and Corynebacterium glutamicum, tatA, tatB, and tatC genes can be identified, suggesting that these organisms, just like E. coli, might use TatABC-type Tat translocases as well. However, since contrary to this view a previous study has suggested that C. glutamicum might in fact use a TatAC translocase with TatB only playing a minor role, we reexamined the requirement of TatB for Tat-dependent protein translocation in this microorganism. Under aerobic conditions, the misassembly of the Rieske iron-sulfur protein QcrA was identified as a major reason for the severe growth defect of Tat-defective C. glutamicum mutant strains. Furthermore, our results clearly show that TatB, besides TatA and TatC, is strictly required for unimpaired aerobic growth. In addition, TatB was also found to be essential for the secretion of a heterologous Tat-dependent model protein into the C. glutamicum culture supernatant. Together with our finding that expression of the C. glutamicum TatB in an E. coli ΔtatB mutant strain resulted in the formation of an active Tat translocase, our results clearly indicate that a TatABC translocase is used as the physiologically relevant functional unit for Tat-dependent protein translocation in C. glutamicum and, most likely, also in other TatB-containing Actinobacteria.
Conflict of interest statement
Figures

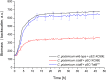

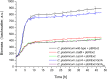


References
-
- Desvaux M, Parham NJ, Scott-Tucker A, Henderson IR. The general secretory pathway: a general misnomer? Trends Microbiol. 2004;12: 306–309. - PubMed
-
- Berks BC. A common export pathway for proteins binding complex redox cofactors? Mol Microbiol. 1996;22: 393–404. - PubMed
-
- Stanley NR, Palmer T, Berks BC. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli . J Biol Chem. 2000;275: 11591–11596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

