DnaK protein alleviates toxicity induced by citrate-coated gold nanoparticles in Escherichia coli
- PMID: 25837593
- PMCID: PMC4383610
- DOI: 10.1371/journal.pone.0121243
DnaK protein alleviates toxicity induced by citrate-coated gold nanoparticles in Escherichia coli
Abstract
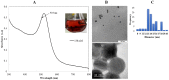
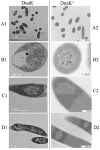
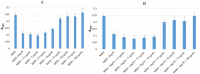
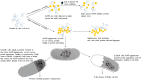
A number of previously reported studies suggest that synthetic gold nanoparticles (AuNPs) are capable of stabilising proteins against heat stress in vitro. However, it remains to be understood if AuNPs confer stability to proteins against cellular stress in vivo. Heat shock proteins (Hsps) are conserved molecules whose main role is to facilitate folding of other proteins (chaperone function). Hsp70 (called DnaK in prokaryotes) is one of the most prominent molecular chaperones. Since gold nanoparticles exhibit chaperone-like function in vitro, we investigated the effect of citrate-coated gold nanoparticles on the growth of E. coli BB1553 cells that possess a deleted dnaK gene. We further investigated the effects of the AuNPs on the solubility of the E. coli BB1553 proteome. E. coli BB1553 cells exposed to AuNPs exhibited cellular defects such as filamentation and plasma membranes pulled off the cell wall. The toxic effects of the AuNPs were alleviated by transforming the E. coli BB1553 cells with a construct expressing DnaK. We also noted that cells in which DnaK was restored exhibited distinct zones to which the nanoparticles were restricted. Our study suggests a role for DnaK in alleviating nanoparticle induced stress in E. coli.
Conflict of interest statement
Figures




Similar articles
-
Complementation studies of the DnaK-DnaJ-GrpE chaperone machineries from Vibrio harveyi and Escherichia coli, both in vivo and in vitro.Arch Microbiol. 2004 Dec;182(6):436-49. doi: 10.1007/s00203-004-0727-8. Epub 2004 Sep 24. Arch Microbiol. 2004. PMID: 15448982
-
Effect of the DnaK chaperone on the conformational quality of JCV VP1 virus-like particles produced in Escherichia coli.Biotechnol Prog. 2014 May-Jun;30(3):744-8. doi: 10.1002/btpr.1879. Epub 2014 Mar 3. Biotechnol Prog. 2014. PMID: 24574306
-
Identification of a gene, closely linked to dnaK, which is required for high-temperature growth of Escherichia coli.J Gen Microbiol. 1991 Jun;137(6):1271-7. doi: 10.1099/00221287-137-6-1271. J Gen Microbiol. 1991. PMID: 1655950
-
In vivo and in vitro complementation study comparing the function of DnaK chaperone systems from halophilic lactic acid bacterium Tetragenococcus halophilus and Escherichia coli.Biosci Biotechnol Biochem. 2008 Mar;72(3):811-22. doi: 10.1271/bbb.70691. Epub 2008 Mar 7. Biosci Biotechnol Biochem. 2008. PMID: 18323638
-
Complementation of an Escherichia coli DnaK defect by Hsc70-DnaK chimeric proteins.J Bacteriol. 2004 Sep;186(18):6248-53. doi: 10.1128/JB.186.18.6248-6253.2004. J Bacteriol. 2004. PMID: 15342595 Free PMC article.
Cited by
-
Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria.Pharmaceuticals (Basel). 2021 Dec 20;14(12):1335. doi: 10.3390/ph14121335. Pharmaceuticals (Basel). 2021. PMID: 34959736 Free PMC article.
-
Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties.Materials (Basel). 2020 Nov 6;13(21):5014. doi: 10.3390/ma13215014. Materials (Basel). 2020. PMID: 33172161 Free PMC article.
-
Silver-coated magnetic nanocomposites induce growth inhibition and protein changes in foodborne bacteria.Sci Rep. 2019 Nov 25;9(1):17499. doi: 10.1038/s41598-019-53080-x. Sci Rep. 2019. PMID: 31767879 Free PMC article.
-
Roles of Hsp70s in Stress Responses of Microorganisms, Plants, and Animals.Biomed Res Int. 2015;2015:510319. doi: 10.1155/2015/510319. Epub 2015 Nov 16. Biomed Res Int. 2015. PMID: 26649306 Free PMC article. Review.
References
-
- Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci. 2007; 167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

