Development of an efficient electroporation method for iturin A-producing Bacillus subtilis ZK
- PMID: 25837631
- PMCID: PMC4425020
- DOI: 10.3390/ijms16047334
Development of an efficient electroporation method for iturin A-producing Bacillus subtilis ZK
Abstract
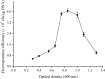
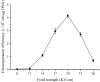
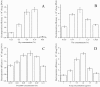
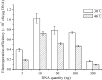
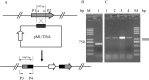
In order to efficiently introduce DNA into B. subtilis ZK, which produces iturin A at a high level, we optimized seven electroporation conditions and explored an efficient electroporation method. Using the optimal conditions, the electroporation efficiency was improved to 1.03 × 10(70 transformants/μg of DNA, an approximately 10,000-fold increase in electroporation efficiency. This efficiency is the highest electroporation efficiency for B. subtilis and enables the construction of a directed evolution library or the knockout of a gene in B. subtilis ZK for molecular genetics studies. In the optimization process, the combined effects of three types of wall-weakening agents were evaluated using a response surface methodology (RSM) design, which led to a two orders of magnitude increase in electroporation efficiency. To the best of our limited knowledge, this study provides the first demonstration of using an RSM design for optimization of the electroporation conditions for B. subtilis. To validate the electroporation efficiency, a case study was performed and a gene (rapC) was inactivated in B. subtilis ZK using a suicide plasmid pMUTIN4. Moreover, we found that the rapC mutants exhibited a marked decrease in iturin A production, suggesting that the rapC gene was closely related to the iturin A production.
Figures

References
-
- Peng W.J., Zhong J., Yang J., Ren Y.L., Xu T., Xiao S., Zhou J.Y., Tan H. The artificial neural network approach based on uniform design to optimize the fed-batch fermentation condition: Application to the production of iturin A. Microb. Cell Fact. 2014;13:54. doi: 10.1186/1475-2859-13-54. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

