Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan
- PMID: 25837762
- PMCID: PMC4554019
- DOI: 10.1097/MD.0000000000000690
Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan
Abstract
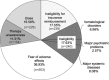
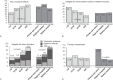
Peginterferon/ribavirin provides a substantially high treatment efficacy for chronic hepatitis C virus (HCV) infections in Asians. Whether the clinical efficacy can be translated to community effectiveness remains unclear. The disease awareness, treatment accessibility, recommendations, acceptance, and barriers to anti-HCV treatment were explored to clarify the issue with a 3-step nationwide investigation in Taiwan. A crude HCV-infected population was estimated using databases from 3 large-scale surveillance studies and age-/geographic-specific population database. HCV awareness and accessibility were investigated at the patient level in 58,129 residents. The recommendations/acceptances and barriers to treatment at the provider level were evaluated using a prospective, nationwide approach to 89 gastroenterologists/hepatologists. The estimated 10-year interval age-adjusted anti-HCV-seropositive population is 745,109 (3.28%), with an anticipated HCV-viremic population of 554,361. Of anti-HCV-seropositive subjects, 36.2% had disease awareness. Among those with awareness, 39.6% had accessibility. The recommendation/acceptance rate of antiviral therapy was 70.6%. The treatment rate was 10.1% and 13.7% for the anti-HCV-seropositive and HCV-viremic population, respectively. With an anticipated treatment success rate of 80% in Taiwan, 8.1% of the anti-HCV-seropositive and 10.9% of the HCV-viremic population achieved successful treatment. The major treatment barriers were fear of adverse effects (37%), major disorders (17.6%), ineligibility for insurance reimbursement (17.6%), and lack of therapy awareness (11.3%). Despite the high rates of treatment response and nationwide coverage of insurance reimbursement, there remains a large gap between clinical efficacy and community effectiveness in anti-HCV treatment in Taiwan. Increasing disease awareness/treatment accessibility and introducing new therapeutic strategies with high tolerability are warranted.
Figures

References
-
- Omata M, Kanda T, Yu ML, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int 2012; 6:409–435. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–1342. - PubMed
-
- Chen CH, Yang PM, Huang GT, et al. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc 2007; 106:148–155. - PubMed
-
- Yu ML, Lee CM, Chen CL, et al. Sustained hepatitis C virus clearance and increased hepatitis B surface antigen seroclearance in patients with dual chronic hepatitis C and B during posttreatment follow-up. Hepatology 2013; 57:2135–2142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

