Immunogenicity, protective efficacy, and non-replicative status of the HSV-2 vaccine candidate HSV529 in mice and guinea pigs
- PMID: 25837802
- PMCID: PMC4383384
- DOI: 10.1371/journal.pone.0121518
Immunogenicity, protective efficacy, and non-replicative status of the HSV-2 vaccine candidate HSV529 in mice and guinea pigs
Abstract
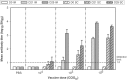
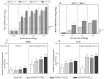
HSV-2 vaccine is needed to prevent genital disease, latent infection, and virus transmission. A replication-deficient mutant virus (dl5-29) has demonstrated promising efficacy in animal models of genital herpes. However, the immunogenicity, protective efficacy, and non-replicative status of the highly purified clinical vaccine candidate (HSV529) derived from dl5-29 have not been evaluated. Humoral and cellular immune responses were measured in mice and guinea pigs immunized with HSV529. Protection against acute and recurrent genital herpes, mortality, latent infection, and viral shedding after vaginal HSV-2 infection was determined in mice or in naïve and HSV-1 seropositive guinea pigs. HSV529 replication and pathogenicity were investigated in three sensitive models of virus replication: severe combined immunodeficient (SCID/Beige) mice inoculated by the intramuscular route, suckling mice inoculated by the intracranial route, and vaginally-inoculated guinea pigs. HSV529 immunization induced HSV-2-neutralizing antibody production in mice and guinea pigs. In mice, it induced production of specific HSV-2 antibodies and splenocytes secreting IFNγ or IL-5. Immunization effectively prevented HSV-2 infection in all three animal models by reducing mortality, acute genital disease severity and frequency, and viral shedding. It also reduced ganglionic viral latency and recurrent disease in naïve and HSV-1 seropositive guinea pigs. HSV529 replication/propagation was not detected in the muscles of SCID/Beige mice, in the brains of suckling mice, or in vaginal secretions of inoculated guinea pigs. These results confirm the non-replicative status, as well as its immunogenicity and efficacy in mice and guinea pigs, including HSV-1 seropositive guinea pigs. In mice, HSV529 produced Th1/Th2 characteristic immune response thought to be necessary for an effective vaccine. These results further support the clinical investigation of HSV529 in human subjects as a prophylactic vaccine.
Conflict of interest statement
Figures




References
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20: 73–83. - PubMed
-
- Wald A, Link K Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185: 45–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

