Comparability of biosimilar filgrastim with originator filgrastim: protein characterization, pharmacodynamics, and pharmacokinetics
- PMID: 25837839
- PMCID: PMC4412827
- DOI: 10.1007/s40259-015-0124-7
Comparability of biosimilar filgrastim with originator filgrastim: protein characterization, pharmacodynamics, and pharmacokinetics
Abstract
Background: Biosimilars provide safety, purity, and potency similar to those of a reference biologic product.
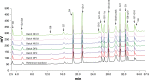
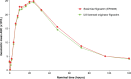
Methods: An array of protein analytical techniques was used to compare the physicochemical properties of proposed biosimilar filgrastim (EP2006), US-approved originator filgrastim, and EU-approved originator filgrastim. Biological characterization involved surface plasmon resonance spectroscopy analyses and in vitro proliferation assays. A randomized, double-blind, two-way crossover, phase I study in healthy volunteers assessed the pharmacodynamics, pharmacokinetics, and safety profiles of EP2006 and US-approved originator filgrastim (administered as a single subcutaneous 10 µg/kg injection).
Results: EP2006 and originator filgrastim (US and EU approved) were highly similar with respect to primary, secondary, and tertiary protein structures; mass, size, purity, charge, and hydrophobicity. No differences in receptor binding affinity were observed, and all samples demonstrated similar in vitro bioactivity. In the phase I study, no statistically significant differences between EP2006 and US-approved originator filgrastim were noted in pharmacodynamic or pharmacokinetic parameters, and all confidence intervals were within the equivalence boundaries. The two products had similar safety profiles.
Conclusion: These studies provide robust evidence of the structural and functional similarity between the proposed biosimilar filgrastim (EP2006) and the US-approved originator filgrastim.
Figures



References
-
- Blackstone EA, Fuhr JP., Jr Innovation and competition: will biosimilars succeed? The creation of an FDA approval pathway for biosimilars is complex and fraught with hazard. Yes, innovation and market competition are at stake. But so are efficacy and patient safety. Biotechnol Healthc. 2012;9:24–27. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

