Next-generation sequencing reveals the biological significance of the N(2),3-ethenoguanine lesion in vivo
- PMID: 25837992
- PMCID: PMC4477646
- DOI: 10.1093/nar/gkv243
Next-generation sequencing reveals the biological significance of the N(2),3-ethenoguanine lesion in vivo
Abstract
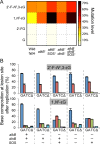
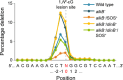
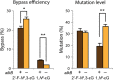
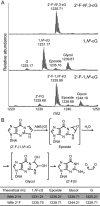
Etheno DNA adducts are a prevalent type of DNA damage caused by vinyl chloride (VC) exposure and oxidative stress. Etheno adducts are mutagenic and may contribute to the initiation of several pathologies; thus, elucidating the pathways by which they induce cellular transformation is critical. Although N(2),3-ethenoguanine (N(2),3-εG) is the most abundant etheno adduct, its biological consequences have not been well characterized in cells due to its labile glycosidic bond. Here, a stabilized 2'-fluoro-2'-deoxyribose analog of N(2),3-εG was used to quantify directly its genotoxicity and mutagenicity. A multiplex method involving next-generation sequencing enabled a large-scale in vivo analysis, in which both N(2),3-εG and its isomer 1,N(2)-ethenoguanine (1,N(2)-εG) were evaluated in various repair and replication backgrounds. We found that N(2),3-εG potently induces G to A transitions, the same mutation previously observed in VC-associated tumors. By contrast, 1,N(2)-εG induces various substitutions and frameshifts. We also found that N(2),3-εG is the only etheno lesion that cannot be repaired by AlkB, which partially explains its persistence. Both εG lesions are strong replication blocks and DinB, a translesion polymerase, facilitates the mutagenic bypass of both lesions. Collectively, our results indicate that N(2),3-εG is a biologically important lesion and may have a functional role in VC-induced or inflammation-driven carcinogenesis.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Abdulla F.R., Feldman S.R., Williford P.M., Krowchuk D., Kaur M. Tanning and skin cancer. Pediatr. Dermatol. 2005;22:501–512. - PubMed
-
- Wogan G.N. Dietary factors and special epidemiological situations of liver cancer in Thailand and Africa. Cancer Res. 1975;35:3499–3502. - PubMed
-
- Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES000267/ES/NIEHS NIH HHS/United States
- P01 CA160032/CA/NCI NIH HHS/United States
- R01 GM069857/GM/NIGMS NIH HHS/United States
- T32 ES007020/ES/NIEHS NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
- R37 CA080024/CA/NCI NIH HHS/United States
- P01 ES005355/ES/NIEHS NIH HHS/United States
- P01 ES05355/ES/NIEHS NIH HHS/United States
- Howard Hughes Medical Institute/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P01 CA026731/CA/NCI NIH HHS/United States
- R01 CA080024/CA/NCI NIH HHS/United States
- S10 RR019022/RR/NCRR NIH HHS/United States
- R01 ES010546/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

