Cytosolic phospholipase A₂: physiological function and role in disease
- PMID: 25838312
- PMCID: PMC4513982
- DOI: 10.1194/jlr.R057588
Cytosolic phospholipase A₂: physiological function and role in disease
Abstract
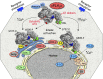
The group IV phospholipase A2 (PLA2) family is comprised of six intracellular enzymes (GIVA, -B, -C, -D, -E, and -F) commonly referred to as cytosolic PLA2 (cPLA2)α, -β, -γ, -δ, -ε, and -ζ. They contain a Ser-Asp catalytic dyad and all except cPLA2γ have a C2 domain, but differences in their catalytic activities and subcellular localization suggest unique regulation and function. With the exception of cPLA2α, the focus of this review, little is known about the in vivo function of group IV enzymes. cPLA2α catalyzes the hydrolysis of phospholipids to arachidonic acid and lysophospholipids that are precursors of numerous bioactive lipids. The regulation of cPLA2α is complex, involving transcriptional and posttranslational processes, particularly increases in calcium and phosphorylation. cPLA2α is a highly conserved widely expressed enzyme that promotes lipid mediator production in human and rodent cells from a variety of tissues. The diverse bioactive lipids produced as a result of cPLA2α activation regulate normal physiological processes and disease pathogenesis in many organ systems, as shown using cPLA2α KO mice. However, humans recently identified with cPLA2α deficiency exhibit more pronounced effects on health than observed in mice lacking cPLA2α, indicating that much remains to be learned about this interesting enzyme.
Keywords: Golgi apparatus; arachidonic acid; calcium; cyclooxygenase; inflammation; leukotrienes; lipoxygenase; prostaglandins; protein kinases/MAP kinase.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Lambeau G., Gelb M. H. 2008. Biochemistry and physiology of mammalian secreted phopholipases A2. Annu. Rev. Biochem. 77: 495–520. - PubMed
-
- Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. 2011. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 50: 152–192. - PubMed
-
- Ghosh M., Tucker D. E., Burchett S. A., Leslie C. C. 2006. Properties of the group IV phospholipase A2 family. Prog. Lipid Res. 45: 487–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

