Pathogenesis beyond the cancer clone(s) in multiple myeloma
- PMID: 25838343
- PMCID: PMC4432002
- DOI: 10.1182/blood-2014-11-568881
Pathogenesis beyond the cancer clone(s) in multiple myeloma
Abstract
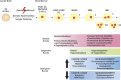
Over the past 4 decades, basic research has provided crucial information regarding the cellular and molecular biology of cancer. In particular, the relevance of cancer microenvironment (including both cellular and noncellular elements) and the concept of clonal evolution and heterogeneity have emerged as important in cancer pathogenesis, immunologic escape, and resistance to therapy. Multiple myeloma (MM), a cancer of terminally differentiated plasma cells, is emblematic of the impact of cancer microenvironment and the role of clonal evolution. Although genetic and epigenetic aberrations occur in MM and evolve over time under the pressure of exogenous stimuli, they are also largely present in premalignant plasma cell dyscrasia such as monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), suggesting that genetic mutations alone are necessary, but not sufficient, for myeloma transformation. The role of bone marrow microenvironment in mediating survival, proliferation, and resistance to therapy in myeloma is well established; and although an appealing speculation, its role in fostering the evolution of MGUS or SMM into MM is yet to be proven. In this review, we discuss MM pathogenesis with a particular emphasis on the role of bone marrow microenvironment.
Figures

References
-
- Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–274. - PubMed
-
- Hauser AE, Muehlinghaus G, Manz RA, et al. Long-lived plasma cells in immunity and inflammation. Ann N Y Acad Sci. 2003;987:266–269. - PubMed
-
- Seifert M, Scholtysik R, Küppers R. Origin and pathogenesis of B cell lymphomas. Methods Mol Biol. 2013;971:1–25. - PubMed
-
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

