New criteria for response assessment: role of minimal residual disease in multiple myeloma
- PMID: 25838346
- PMCID: PMC4513329
- DOI: 10.1182/blood-2014-11-568907
New criteria for response assessment: role of minimal residual disease in multiple myeloma
Abstract
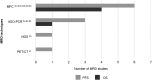
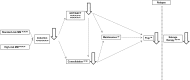
Assessment of minimal residual disease (MRD) is becoming standard diagnostic care for potentially curable neoplasms such as acute lymphoblastic leukemia. In multiple myeloma (MM), the majority of patients will inevitably relapse despite achievement of progressively higher complete remission (CR) rates. Novel treatment protocols with inclusion of antibodies and small molecules might well be able to further increase remission rates and potentially also cure rates. Therefore, MRD diagnostics becomes essential to assess treatment effectiveness. This review summarizes reports from the past 2 decades, which demonstrate that persistent MRD by multiparameter flow cytometry, polymerase chain reaction, next-generation sequencing, and positron emission tomography/computed tomography, predicts significantly inferior survival among CR patients. We describe the specific features of currently available techniques for MRD monitoring and outline the arguments favoring new criteria for response assessment that incorporate MRD levels. Extensive data indicate that MRD information can potentially be used as biomarker to evaluate the efficacy of different treatment strategies, help on treatment decisions, and act as surrogate for overall survival. The time has come to address within clinical trials the exact role of baseline risk factors and MRD monitoring for tailored therapy in MM, which implies systematic usage of highly sensitive, cost-effective, readily available, and standardized MRD techniques.
© 2015 by The American Society of Hematology.
Figures


References
-
- Cavo M, Pantani L, Petrucci MT, et al. GIMEMA (Gruppo Italiano Malattie Ematologiche dell’Adulto) Italian Myeloma Network. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120(1):9–19. - PubMed
-
- Mateos MV, Oriol A, Martinez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887–1893. - PubMed
-
- Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

