Adoptive cell transfer as personalized immunotherapy for human cancer
- PMID: 25838374
- PMCID: PMC6295668
- DOI: 10.1126/science.aaa4967
Adoptive cell transfer as personalized immunotherapy for human cancer
Abstract
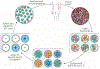
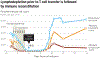
Adoptive cell therapy (ACT) is a highly personalized cancer therapy that involves administration to the cancer-bearing host of immune cells with direct anticancer activity. ACT using naturally occurring tumor-reactive lymphocytes has mediated durable, complete regressions in patients with melanoma, probably by targeting somatic mutations exclusive to each cancer. These results have expanded the reach of ACT to the treatment of common epithelial cancers. In addition, the ability to genetically engineer lymphocytes to express conventional T cell receptors or chimeric antigen receptors has further extended the successful application of ACT for cancer treatment.
Copyright © 2015, American Association for the Advancement of Science.
Figures
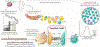
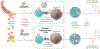
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

