Epidermal tight junctions in health and disease
- PMID: 25838981
- PMCID: PMC4372028
- DOI: 10.4161/21688370.2014.974451
Epidermal tight junctions in health and disease
Abstract
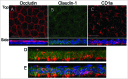
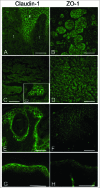
The skin, the largest organ of the body, is an essential barrier that under homeostatic conditions efficiently protects and/or minimizes damage from both environmental (e.g. microorganisms, physical trauma, ultraviolet radiation) and endogenous (e.g., cancers, inflammation) factors. This formidable barrier function resides mainly in the epidermis, a dynamic, highly-stratified epithelium. The epidermis has 2 major barrier structures: stratum corneum, the outmost layer and tight junctions, intercellular junctions that seal adjacent keratinocytes in the stratum granulosum, found below the stratum corneum. In recent years there have been significant advances in our understanding of tight junction function, composition and regulation. Herein we review what is known about tight junctions in healthy skin and keratinocyte culture systems and highlight the dynamic crosstalk observed between tight junctions and the cutaneous immune system. Finally we discuss the preliminary observations suggesting that tight junction function or protein expression may be relevant for the pathogenesis of a number of common cutaneous inflammatory and neoplastic conditions.
Keywords: AD, atopic dermatitis; AMP, antimicrobial peptides; Cldn, claudin; DC, dendritic cells; FLG, filaggrin; JAM, junctional adhesion molecule; LC, Langerhans cells; MM, malignant melanoma; PRR, pattern recognition receptor; PS, psoriasis; SCC, squamous cell carcinoma; SC, stratum corneum; SG, stratum granulosum; SNP, single nucleotide polymorphism; TER, TransEpithelial Electrical Resistance; TJ, tight junction; TLR, Toll-like receptor; Th, T helper; ZO-1, zonula occludens 1; claudins; skin barrier; skin immune system; skin innate barrier; tight junction.
Figures
References
-
- Chu D.H. HAR, Holbrook K, Loomis C. A. Fitzpatrick's Dermatology in General Medicine. McGraw-Hill Companies, Inc; 6(1):58-88.
-
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol 2009; 1:a002584; PMID:; http://dx.doi.org/ 10.1101/cshperspect.a002584 - DOI - PMC - PubMed
-
- Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 2009; 206:2937-46; PMID:; http://dx.doi.org/ 10.1084/jem.20091527 - DOI - PMC - PubMed
-
- Takano K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, Sawada N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. Journal Histochem Cytochem: Off J Histochem Soc 2005; 53:611-9; PMID:; http://dx.doi.org/ 10.1369/jhc.4A6539.2005 - DOI - PubMed
-
- Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol 2003; 121:231-42; PMID:; http://dx.doi.org/ 10.1046/j.1523-1747.2003.12359.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
