Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells
- PMID: 25839988
- PMCID: PMC4454887
- DOI: 10.1038/bjc.2015.113
Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells
Abstract
Background: In recent years, the rapidly advancing field of low-temperature atmospheric pressure plasmas has shown considerable promise for future translational biomedical applications, including cancer therapy, through the generation of reactive oxygen and nitrogen species.
Method: The cytopathic effect of low-temperature plasma was first verified in two commonly used prostate cell lines: BPH-1 and PC-3 cells. The study was then extended to analyse the effects in paired normal and tumour (Gleason grade 7) prostate epithelial cells cultured directly from patient tissue. Hydrogen peroxide (H2O2) and staurosporine were used as controls throughout.
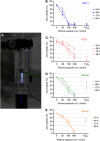
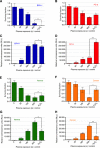
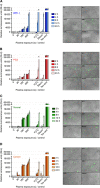
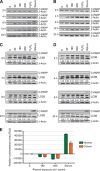
Results: Low-temperature plasma (LTP) exposure resulted in high levels of DNA damage, a reduction in cell viability, and colony-forming ability. H2O2 formed in the culture medium was a likely facilitator of these effects. Necrosis and autophagy were recorded in primary cells, whereas cell lines exhibited apoptosis and necrosis.
Conclusions: This study demonstrates that LTP treatment causes cytotoxic insult in primary prostate cells, leading to rapid necrotic cell death. It also highlights the need to study primary cultures in order to gain more realistic insight into patient response.
Figures


References
-
- Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci. 1999;77:658–666.
-
- Casiano CA, Ochs RL, Tan EM. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 1998;5:183–190. - PubMed
-
- Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, Lee JS, Moon E, Baek SJ, Lee K, Kim CH. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys. 2014;545:133–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

