In Vivo Suppression of Heat Shock Protein (HSP)27 and HSP70 Accelerates DMBA-Induced Skin Carcinogenesis by Inducing Antigenic Unresponsiveness to the Initiating Carcinogenic Chemical
- PMID: 25840912
- PMCID: PMC4648556
- DOI: 10.4049/jimmunol.1402804
In Vivo Suppression of Heat Shock Protein (HSP)27 and HSP70 Accelerates DMBA-Induced Skin Carcinogenesis by Inducing Antigenic Unresponsiveness to the Initiating Carcinogenic Chemical
Abstract
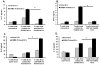
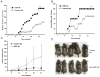
Heat shock proteins (HSPs) are constitutively expressed in murine skin. HSP27 is present in the epidermis, and HSP70 can be found in both the epidermis and dermis. The purpose of this study was to investigate the role of these proteins in cutaneous chemical carcinogenesis and to determine whether their effects on cell-mediated immune function were a contributing factor. In vivo inhibition of HSP27 and HSP70 produced a reduction in the T cell-mediated immune response to 7,12-dimethylbenz(a)anthracene (DMBA) and benzo(a)pyrene in C3H/HeN mice and resulted in a state of Ag-specific tolerance. When mice were pretreated with anti-HSP27 and anti-HSP70 Abs in vivo prior to subjecting them to a standard two-stage DMBA/12-O-tetradecanoylphorbol-13-acetate cutaneous carcinogenesis protocol, the percentage of mice with tumors was much greater (p < 0.05) in anti-HSP27- and HSP70-pretreated animals compared with mice pretreated with control Ab. Similar results were obtained when the data were evaluated as the cumulative number of tumors per group. Mice pretreated with HSP27 and HSP70 Abs developed more H-ras mutations and fewer DMBA-specific cytotoxic T lymphocytes. These findings indicate that in mice HSP27 and HSP70 play a key role in the induction of cell-mediated immunity to carcinogenic polyaromatic hydrocarbons. Bolstering the immune response to carcinogenic polyaromatic hydrocarbons may be an effective method for prevention of the tumors that they produce.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures



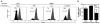
Similar articles
-
Heat shock proteins HSP27 and HSP70 are present in the skin and are important mediators of allergic contact hypersensitivity.J Immunol. 2009 Jan 1;182(1):675-83. doi: 10.4049/jimmunol.182.1.675. J Immunol. 2009. PMID: 19109201 Free PMC article.
-
Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis.Cancer Res. 2008 Jan 15;68(2):615-22. doi: 10.1158/0008-5472.CAN-07-5219. Cancer Res. 2008. PMID: 18199559 Free PMC article.
-
Susceptibility to the biological effects of polyaromatic hydrocarbons is influenced by genes of the major histocompatibility complex.Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14915-9. doi: 10.1073/pnas.95.25.14915. Proc Natl Acad Sci U S A. 1998. PMID: 9843990 Free PMC article.
-
Host defense mechanisms in polyaromatic hydrocarbon carcinogenesis.Skin Pharmacol Appl Skin Physiol. 2001 Nov-Dec;14(6):386-92. doi: 10.1159/000056372. Skin Pharmacol Appl Skin Physiol. 2001. PMID: 11598438 Review.
-
Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties.Cell Cycle. 2006 Nov;5(22):2592-601. doi: 10.4161/cc.5.22.3448. Epub 2006 Nov 15. Cell Cycle. 2006. PMID: 17106261 Review.
Cited by
-
Altered Hypoxia-Induced and Heat Shock Protein Immunostaining in Secondary Hair Follicles Associated with Changes in Altitude and Temperature in Tibetan Cashmere Goats.Animals (Basel). 2021 Sep 25;11(10):2798. doi: 10.3390/ani11102798. Animals (Basel). 2021. PMID: 34679820 Free PMC article.
-
Heat shock proteins in the physiology and pathophysiology of epidermal keratinocytes.Cell Stress Chaperones. 2019 Nov;24(6):1027-1044. doi: 10.1007/s12192-019-01044-5. Epub 2019 Nov 16. Cell Stress Chaperones. 2019. PMID: 31734893 Free PMC article. Review.
-
Murine Skin Carcinogenesis and the Role of Immune System Dysregulation in the Tumorigenicity of 2-Ethylhexyl Acrylate.Biomed Hub. 2020 Sep 2;5(2):958-973. doi: 10.1159/000508295. eCollection 2020 May-Aug. Biomed Hub. 2020. PMID: 33564662 Free PMC article. Review.
-
Balance between Health Risks and Benefits for Outdoor Workers Exposed to Solar Radiation: An Overview on the Role of Near Infrared Radiation Alone and in Combination with Other Solar Spectral Bands.Int J Environ Res Public Health. 2020 Feb 20;17(4):1357. doi: 10.3390/ijerph17041357. Int J Environ Res Public Health. 2020. PMID: 32093162 Free PMC article. Review.
-
Radix et Rhizoma Ginseng chemoprevents both initiation and promotion of cutaneous carcinoma by enhancing cell-mediated immunity and maintaining redox homeostasis.J Ginseng Res. 2020 Jul;44(4):580-592. doi: 10.1016/j.jgr.2019.05.004. Epub 2019 May 21. J Ginseng Res. 2020. PMID: 32617038 Free PMC article.
References
-
- Young JC, V, Agashe R, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nature reviews Molecular cell biology. 2004;5:781–791. - PubMed
-
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

