Leptin-STAT3-G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression
- PMID: 25840984
- PMCID: PMC4694051
- DOI: 10.1158/0008-5472.CAN-14-3076
Leptin-STAT3-G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression
Abstract
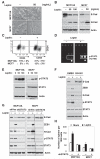
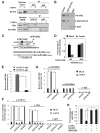
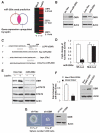
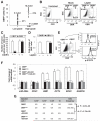
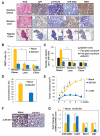
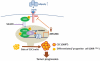
Obesity has been linked to breast cancer progression but the underlying mechanisms remain obscure. Here we report how leptin, an obesity-associated adipokine, regulates a transcriptional pathway to silence a genetic program of epithelial homeostasis in breast cancer stem-like cells (CSC) that promotes malignant progression. Using genome-wide ChIP-seq and RNA expression profiling, we defined a role for activated STAT3 and G9a histone methyltransferase in epigenetic silencing of miR-200c, which promotes the formation of breast CSCs defined by elevated cell surface levels of the leptin receptor (OBR(hi)). Inhibiting the STAT3/G9a pathway restored expression of miR-200c, which in turn reversed the CSC phenotype to a more differentiated epithelial phenotype. In a rat model of breast cancer driven by diet-induced obesity, STAT3 blockade suppressed the CSC-like OBR(hi) population and abrogated tumor progression. Together, our results show how targeting STAT3-G9a signaling regulates CSC plasticity during obesity-related breast cancer progression, suggesting a novel therapeutic paradigm to suppress CSC pools and limit breast malignancy.
©2015 American Association for Cancer Research.
Figures

Comment in
-
Findings of Research Misconduct.Fed Regist. 2022 Dec 16;87(241):77128-77129. Fed Regist. 2022. PMID: 36561292 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

