TGF-β-induced IL-6 prevents development of acute lung injury in influenza A virus-infected F508del CFTR-heterozygous mice
- PMID: 25840995
- PMCID: PMC4451396
- DOI: 10.1152/ajplung.00078.2015
TGF-β-induced IL-6 prevents development of acute lung injury in influenza A virus-infected F508del CFTR-heterozygous mice
Abstract
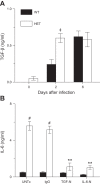
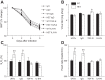
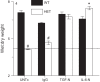
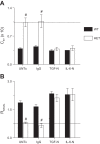
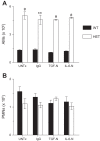
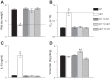
As the eighth leading cause of annual mortality in the USA, influenza A viruses are a major public health concern. In 20% of patients, severe influenza progresses to acute lung injury (ALI). However, pathophysiological mechanisms underlying ALI development are poorly defined. We reported that, unlike wild-type (WT) C57BL/6 controls, influenza A virus-infected mice that are heterozygous for the F508del mutation in the cystic fibrosis transmembrane conductance regulator (HETs) did not develop ALI. This effect was associated with higher IL-6 and alveolar macrophages (AMs) at 6 days postinfection (d.p.i.) in HET bronchoalveolar lavage fluid (BALF). In the present study, we found that HET AMs were an important source of IL-6 at 6 d.p.i. Infection also induced TGF-β production by HET but not WT mice at 2 d.p.i. TGF-β neutralization at 2 d.p.i. (TGF-N) significantly reduced BALF IL-6 in HETs at 6 d.p.i. Neither TGF-N nor IL-6 neutralization at 4 d.p.i. (IL-6-N) altered postinfection weight loss or viral replication in either mouse strain. However, both treatments increased influenza A virus-induced hypoxemia, pulmonary edema, and lung dysfunction in HETs to WT levels at 6 d.p.i. TGF-N and IL-6-N did not affect BALF AM and neutrophil numbers but attenuated the CXCL-1/keratinocyte chemokine response in both strains and reduced IFN-γ production in WT mice. Finally, bone marrow transfer experiments showed that HET stromal and myeloid cells are both required for protection from ALI in HETs. These findings indicate that TGF-β-dependent production of IL-6 by AMs later in infection prevents ALI development in influenza A virus-infected HET mice.
Keywords: acute lung injury; cystic fibrosis transmembrane conductance regulator; influenza; interleukin-6; transforming growth factor-β.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Ecto-5'-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2015 Dec 1;309(11):L1313-22. doi: 10.1152/ajplung.00130.2015. Epub 2015 Oct 2. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26432867 Free PMC article.
-
Heterozygosity for the F508del mutation in the cystic fibrosis transmembrane conductance regulator anion channel attenuates influenza severity.J Infect Dis. 2013 Sep 1;208(5):780-9. doi: 10.1093/infdis/jit251. Epub 2013 Jun 7. J Infect Dis. 2013. PMID: 23749967 Free PMC article.
-
Activation of A1-adenosine receptors promotes leukocyte recruitment to the lung and attenuates acute lung injury in mice infected with influenza A/WSN/33 (H1N1) virus.J Virol. 2014 Sep 1;88(17):10214-27. doi: 10.1128/JVI.01068-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965449 Free PMC article.
-
Bleomycin-Induced Lung Injury Increases Resistance to Influenza Virus Infection in a Type I Interferon-Dependent Manner.Front Immunol. 2021 Aug 18;12:697162. doi: 10.3389/fimmu.2021.697162. eCollection 2021. Front Immunol. 2021. PMID: 34484196 Free PMC article.
-
Alveolar Epithelial Cells Promote IGF-1 Production by Alveolar Macrophages Through TGF-β to Suppress Endogenous Inflammatory Signals.Front Immunol. 2020 Jul 21;11:1585. doi: 10.3389/fimmu.2020.01585. eCollection 2020. Front Immunol. 2020. PMID: 32793225 Free PMC article.
Cited by
-
LAP+ Cells Modulate Protection Induced by Oral Vaccination with Rhesus Rotavirus in a Neonatal Mouse Model.J Virol. 2019 Sep 12;93(19):e00882-19. doi: 10.1128/JVI.00882-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292251 Free PMC article.
-
Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection.Mucosal Immunol. 2018 Mar;11(2):523-535. doi: 10.1038/mi.2017.77. Epub 2017 Oct 25. Mucosal Immunol. 2018. PMID: 29067998 Free PMC article.
-
X-Box-Binding Protein 1 and Innate Immune Responses of Human Cystic Fibrosis Alveolar Macrophages.Am J Respir Crit Care Med. 2015 Dec 15;192(12):1449-61. doi: 10.1164/rccm.201504-0657OC. Am J Respir Crit Care Med. 2015. PMID: 26331676 Free PMC article.
-
Human lung ex vivo infection models.Cell Tissue Res. 2017 Mar;367(3):511-524. doi: 10.1007/s00441-016-2546-z. Epub 2016 Dec 20. Cell Tissue Res. 2017. PMID: 27999962 Free PMC article. Review.
-
Aromatic amino acid metabolites alter interferon signaling and influenza pathogenesis.Front Mol Biosci. 2024 Jan 23;10:1232573. doi: 10.3389/fmolb.2023.1232573. eCollection 2023. Front Mol Biosci. 2024. PMID: 38322710 Free PMC article.
References
-
- Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Sugano K. Existence of autocrine loop between interleukin-6 and transforming growth factor-β1 in activated rat pancreatic stellate cells. J Cell Biochem 99: 221–228, 2006. - PubMed
-
- Boucher RC, Chinet T, Willumsen N, Knowles MR, Stutts MJ. Ion transport in normal and CF airway epithelia. Adv Exp Med Biol 290: 105–115, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

