Role of mitochondrial fission and fusion in cardiomyocyte contractility
- PMID: 25841124
- PMCID: PMC4442057
- DOI: 10.1016/j.ijcard.2015.03.352
Role of mitochondrial fission and fusion in cardiomyocyte contractility
Abstract
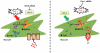
Background: Mitochondria constitute 30% of cell volume and are engaged in two dynamic processes called fission and fusion, regulated by Drp-1 (dynamin related protein) and mitofusin 2 (Mfn2). Previously, we showed that Drp-1 inhibition attenuates cardiovascular dysfunction following pressure overload in aortic banding model and myocardial infarction. As dynamic organelles, mitochondria are capable of changing their morphology in response to stress. However, whether such changes can alter their function and in turn cellular function is unknown. Further, a direct role of fission and fusion in cardiomyocyte contractility has not yet been studied. In this study, we hypothesize that disrupted fission and fusion balance by increased Drp-1 and decreased Mfn2 expression in cardiomyocytes affects their contractility through alterations in the calcium and potassium concentrations.
Methods: To verify this, we used freshly isolated ventricular myocytes from wild type mouse and transfected them with either siRNA to Drp-1 or Mfn2. Myocyte contractility studies were performed by IonOptix using a myopacer. Intracellular calcium and potassium measurements were done using flow cytometry. Immunocytochemistry (ICC) was done to evaluate live cell mitochondria and its membrane potential. Protein expression was done by western blot and immunocytochemistry.
Results: We found that silencing mitochondrial fission increased the myocyte contractility, while fusion inhibition decreased contractility with simultaneous changes in calcium and potassium. Also, we observed that increase in fission prompted decrease in Serca-2a and increase in cytochrome c leakage leading to mitophagy.
Conclusion: Our results suggested that regulating mitochondrial fission and fusion have direct effects on overall cardiomyocyte contractility and thus function.
Keywords: Calcium; Cytochrome c; Drp-1; Heart; Mfn2; Mitochondria; Serca-2a.
Copyright © 2015. Published by Elsevier Ireland Ltd.
Figures











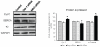
References
-
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J.Clin.Invest. 2000;106:55–62. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

