Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice
- PMID: 25842287
- PMCID: PMC4591101
- DOI: 10.1016/j.jaci.2015.02.031
Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice
Abstract
Background: Asthma, a chronic inflammatory condition defined by episodic shortness of breath with expiratory wheezing and cough, is a serious health concern affecting more than 250 million persons. Genome-wide association studies have identified ORM (yeast)-like protein isoform 3 (ORMDL3) as a gene associated with susceptibility to asthma. Although its yeast ortholog is a negative regulator of de novo ceramide biosynthesis, how ORMDL3 contributes to asthma pathogenesis is not known.
Objectives: We sought to decipher the molecular mechanism for the pathologic functions of ORMDL3 in asthma and the relationship to its evolutionarily conserved role in regulation of ceramide homeostasis.
Methods: We determined the relationship between expression of ORMDL3 and ceramide in epithelial and inflammatory cells and in asthma pathogenesis in mice.
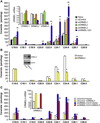
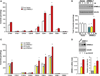
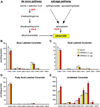
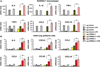
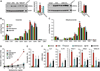
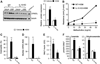
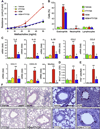
Results: Although small increases in ORMDL3 expression decrease ceramide levels, remarkably, higher expression in lung epithelial cells and macrophages in vitro and in vivo increased ceramide production, which promoted chronic inflammation, airway hyperresponsiveness, and mucus production during house dust mite-induced allergic asthma. Moreover, nasal administration of the immunosuppressant drug FTY720/fingolimod reduced ORMDL3 expression and ceramide levels and mitigated airway inflammation and hyperreactivity and mucus hypersecretion in house dust mite-challenged mice.
Conclusions: Our findings demonstrate that overexpression of ORMDL3 regulates ceramide homeostasis in cells in a complex manner and suggest that local FTY720 administration might be a useful therapeutic intervention for the control of allergic asthma.
Keywords: Asthma; FTY720; ORMDL3; ceramide; house dust mite; sphingolipid.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: S. Spiegel, D. H. Conrad, A. Monpetit, and J. Newton have been supported by grants from the National Institutes of Health (R37GM043880, R01AI50094, U19AI077435, R00NR012016, T32HL094290). The rest of the authors declare that they have no relevant conflicts of interest.
Figures

Similar articles
-
ORMDL3 and allergic asthma: From physiology to pathology.J Allergy Clin Immunol. 2019 Sep;144(3):634-640. doi: 10.1016/j.jaci.2019.07.023. Epub 2019 Jul 31. J Allergy Clin Immunol. 2019. PMID: 31376405 Free PMC article. Review.
-
The ORMDL3 asthma susceptibility gene regulates systemic ceramide levels without altering key asthma features in mice.J Allergy Clin Immunol. 2019 Dec;144(6):1648-1659.e9. doi: 10.1016/j.jaci.2019.06.041. Epub 2019 Jul 20. J Allergy Clin Immunol. 2019. PMID: 31330218 Free PMC article.
-
Treatment of Allergic Asthma with Fenretinide Formulation (LAU-7b) Downregulates ORMDL Sphingolipid Biosynthesis Regulator 3 (Ormdl3) Expression and Normalizes Ceramide Imbalance.J Pharmacol Exp Ther. 2020 Jun;373(3):476-487. doi: 10.1124/jpet.119.263715. Epub 2020 Apr 9. J Pharmacol Exp Ther. 2020. PMID: 32273303
-
Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease.J Allergy Clin Immunol. 2017 May;139(5):1496-1507.e3. doi: 10.1016/j.jaci.2016.07.033. Epub 2016 Sep 10. J Allergy Clin Immunol. 2017. PMID: 27623174 Free PMC article.
-
Sphingolipids, ORMDL3 and asthma: what is the evidence?Curr Opin Clin Nutr Metab Care. 2017 Mar;20(2):99-103. doi: 10.1097/MCO.0000000000000349. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28030368 Review.
Cited by
-
1-Deoxysphingolipids Encountered Exogenously and Made de Novo: Dangerous Mysteries inside an Enigma.J Biol Chem. 2015 Jun 19;290(25):15380-15389. doi: 10.1074/jbc.R115.658823. Epub 2015 May 6. J Biol Chem. 2015. PMID: 25947379 Free PMC article. Review.
-
ORMDL3 and allergic asthma: From physiology to pathology.J Allergy Clin Immunol. 2019 Sep;144(3):634-640. doi: 10.1016/j.jaci.2019.07.023. Epub 2019 Jul 31. J Allergy Clin Immunol. 2019. PMID: 31376405 Free PMC article. Review.
-
The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: Reconstitution of SPT regulation in isolated membranes.J Biol Chem. 2019 Mar 29;294(13):5146-5156. doi: 10.1074/jbc.RA118.007291. Epub 2019 Jan 30. J Biol Chem. 2019. PMID: 30700557 Free PMC article.
-
Loss of the zona pellucida-binding protein 2 (Zpbp2) gene in mice impacts airway hypersensitivity and lung lipid metabolism in a sex-dependent fashion.Mamm Genome. 2018 Apr;29(3-4):281-298. doi: 10.1007/s00335-018-9743-x. Epub 2018 Mar 13. Mamm Genome. 2018. PMID: 29536159
-
Negative regulatory roles of ORMDL3 in the FcεRI-triggered expression of proinflammatory mediators and chemotactic response in murine mast cells.Cell Mol Life Sci. 2016 Mar;73(6):1265-85. doi: 10.1007/s00018-015-2047-3. Epub 2015 Sep 25. Cell Mol Life Sci. 2016. PMID: 26407610 Free PMC article.
References
-
- Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. - PubMed
-
- Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

