Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches
- PMID: 25842978
- PMCID: PMC4387376
- DOI: 10.1016/j.stem.2015.02.006
Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches
Abstract
Basal cell carcinoma (BCC) is characterized by frequent loss of PTCH1, leading to constitutive activation of the Hedgehog pathway. Although the requirement for Hedgehog in BCC is well established, the identity of disease-initiating cells and the compartments in which they reside remain controversial. By using several inducible Cre drivers to delete Ptch1 in different cell compartments in mice, we show here that multiple hair follicle stem cell populations readily develop BCC-like tumors. In contrast, stem cells within the interfollicular epidermis do not efficiently form tumors. Notably, we observed that innervated Gli1-expressing progenitors within mechanosensory touch dome epithelia are highly tumorigenic. Sensory nerves activate Hedgehog signaling in normal touch domes, while denervation attenuates touch dome-derived tumors. Together, our studies identify varying tumor susceptibilities among different stem cell populations in the skin, highlight touch dome epithelia as "hot spots" for tumor formation, and implicate cutaneous nerves as mediators of tumorigenesis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

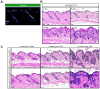

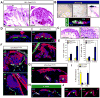

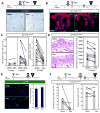
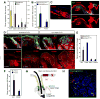
Comment in
-
Tumorigenesis: A hairy and nervous start.Nat Rev Cancer. 2015 May;15(5):257. doi: 10.1038/nrc3952. Nat Rev Cancer. 2015. PMID: 25907214 No abstract available.
References
-
- Adolphe C, Nieuwenhuis E, Villani R, Li ZJ, Kaur P, Hui CC, Wainwright B. Patched1 and Patched2 redundancy plays a key role in regulating epidermal differentiation. J Invest Dermatol. 2014;134:1981–1990. - PubMed
-
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
-
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. - PubMed
-
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, Westendorp RG, Vermeer BJ, Bouwes Bavinck JN. Differences in age, site distribution, and sex between nodular and superficial basal cell carcinoma indicate different types of tumors. J Invest Dermatol. 1998;110:880–884. - PubMed
-
- Berton TR, Matsumoto T, Page A, Conti CJ, Deng CX, Jorcano JL, Johnson DG. Tumor formation in mice with conditional inactivation of Brca1 in epithelial tissues. Oncogene. 2003;22:5415–5426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA046592/CA/NCI NIH HHS/United States
- R01CA087837/CA/NCI NIH HHS/United States
- R21 AR063852/AR/NIAMS NIH HHS/United States
- R00AR059796/AR/NIAMS NIH HHS/United States
- R01AR063437/AR/NIAMS NIH HHS/United States
- R01AR045973/AR/NIAMS NIH HHS/United States
- R00 AR059796/AR/NIAMS NIH HHS/United States
- K99 AR059796/AR/NIAMS NIH HHS/United States
- R01AR065409/AR/NIAMS NIH HHS/United States
- R01 AR045973/AR/NIAMS NIH HHS/United States
- R01 CA087837/CA/NCI NIH HHS/United States
- R01 AR063437/AR/NIAMS NIH HHS/United States
- R01 AR062546/AR/NIAMS NIH HHS/United States
- R21AR063852/AR/NIAMS NIH HHS/United States
- R01 AR065409/AR/NIAMS NIH HHS/United States
- T32 GM007315/GM/NIGMS NIH HHS/United States
- R01AR062546/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

