In Vivo Tumor Vasculature Targeting of CuS@MSN Based Theranostic Nanomedicine
- PMID: 25843647
- PMCID: PMC4414921
- DOI: 10.1021/nn507241v
In Vivo Tumor Vasculature Targeting of CuS@MSN Based Theranostic Nanomedicine
Abstract
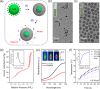
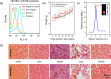
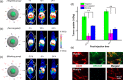
Actively targeted theranostic nanomedicine may be the key for future personalized cancer management. Although numerous types of theranostic nanoparticles have been developed in the past decade for cancer treatment, challenges still exist in the engineering of biocompatible theranostic nanoparticles with highly specific in vivo tumor targeting capabilities. Here, we report the design, synthesis, surface engineering, and in vivo active vasculature targeting of a new category of theranostic nanoparticle for future cancer management. Water-soluble photothermally sensitive copper sulfide nanoparticles were encapsulated in biocompatible mesoporous silica shells, followed by multistep surface engineering to form the final theranostic nanoparticles. Systematic in vitro targeting, an in vivo long-term toxicity study, photothermal ablation evaluation, in vivo vasculature targeted imaging, biodistribution and histology studies were performed to fully explore the potential of as-developed new theranostic nanoparticles.
Keywords: CuS; mesoporous silica nanoparticle; photothermal ablation; theranostic nanomedicine; vasculature targeting.
Figures

References
-
- Blackwell K. L.; Burstein H. J.; Storniolo A. M.; Rugo H. S.; Sledge G.; Aktan G.; Ellis C.; Florance A.; Vukelja S.; Bischoff J.; et al. Overall Survival Benefit with Lapatinib in Combination with Trastuzumab for Patients with Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer: Final Results from the EGF104900 Study. J. Clin. Oncol. 2012, 30, 2585–2592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

