Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle
- PMID: 25843709
- PMCID: PMC4394047
- DOI: 10.1016/j.celrep.2015.03.011
Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle
Abstract
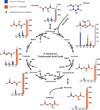
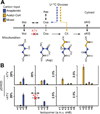
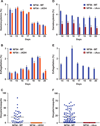
New antimalarial drugs are urgently needed to control drug-resistant forms of the malaria parasite Plasmodium falciparum. Mitochondrial electron transport is the target of both existing and new antimalarials. Herein, we describe 11 genetic knockout (KO) lines that delete six of the eight mitochondrial tricarboxylic acid (TCA) cycle enzymes. Although all TCA KOs grew normally in asexual blood stages, these metabolic deficiencies halted life-cycle progression in later stages. Specifically, aconitase KO parasites arrested as late gametocytes, whereas α-ketoglutarate-dehydrogenase-deficient parasites failed to develop oocysts in the mosquitoes. Mass spectrometry analysis of (13)C-isotope-labeled TCA mutant parasites showed that P. falciparum has significant flexibility in TCA metabolism. This flexibility manifested itself through changes in pathway fluxes and through altered exchange of substrates between cytosolic and mitochondrial pools. Our findings suggest that mitochondrial metabolic plasticity is essential for parasite development.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

