Serodiagnosis for tumor viruses
- PMID: 25843726
- PMCID: PMC4387312
- DOI: 10.1053/j.seminoncol.2014.12.024
Serodiagnosis for tumor viruses
Abstract
The known human tumor viruses include the DNA viruses Epstein-Barr virus (EBV), Kaposi sarcoma herpesvirus (KSHV), Merkel cell polyomavirus (MCPyV), human papillomavirus (HPV), and hepatitis B virus (BV). RNA tumor viruses include human T-cell lymphotrophic virus type 1 (HTLV-1) and hepatitis C virus (HCV). The serological identification of antigens/antibodies in serum is a rapidly progressing field with utility for both scientists and clinicians. Serology is useful for conducting seroepidemiology studies and to inform on the pathogenesis and host immune response to a particular viral agent. Clinically, serology is useful for diagnosing current or past infection and for aiding in clinical management decisions. Serology is useful for screening blood donations for infectious agents and for monitoring the outcome of vaccination against these viruses. Serodiagnosis of human tumor viruses has improved in recent years with increased specificity and sensitivity of the assays, as well as reductions in cost and the ability to assess multiple antibody/antigens in single assays. Serodiagnosis of tumor viruses plays an important role in our understanding of the prevalence and transmission of these viruses and ultimately in the ability to develop treatments/preventions for these globally important diseases.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
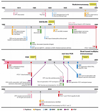
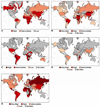
References
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer Journal international du cancer. 2006;118(12):3030–3044. - PubMed
-
- Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon, France, 17–24 June 1997. IARC Monogr Eval Carcinog Risks Hum. 1997;70:1–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

