Protein derivatization and sequential ion/ion reactions to enhance sequence coverage produced by electron transfer dissociation mass spectrometry
- PMID: 25844056
- PMCID: PMC4379715
- DOI: 10.1016/j.ijms.2014.06.023
Protein derivatization and sequential ion/ion reactions to enhance sequence coverage produced by electron transfer dissociation mass spectrometry
Abstract
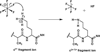
Previously, we described implementation of a front-end ETD (electron transfer dissociation) source for an Orbitrap instrument (1). This source facilitates multiple fills of the C-trap with product ions from ETD of intact proteins prior to mass analysis. The result is a dramatic enhancement of the observed ion current without the need for time consuming averaging of data from multiple mass measurements. Here we show that ion-ion proton transfer (IIPT) reactions can be used to simplify ETD spectra and to disperse fragment ions over the entire mass range in a controlled manner. We also show that protein derivatization can be employed to selectively enhance the sequence information observed at the N- and C-termini of a protein.
Keywords: ETD; IIPT; protein derivatization.
Figures







References
-
- Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J Am Chem Soc. 1998;120(13):3265–3266. 04/01; 2014/02;
-
- Garcia BA. What does the future hold for top down mass spectrometry? J Am Soc Mass Spectrom. 2010;21:193–202. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
