Multiscale modelling of the feto-placental vasculature
- PMID: 25844150
- PMCID: PMC4342946
- DOI: 10.1098/rsfs.2014.0078
Multiscale modelling of the feto-placental vasculature
Abstract
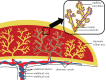
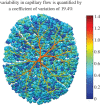
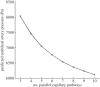
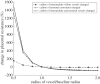
The placenta provides all the nutrients required for the fetus through pregnancy. It develops dynamically, and, to avoid rejection of the fetus, there is no mixing of fetal and maternal blood; rather, the branched placental villi 'bathe' in blood supplied from the uterine arteries. Within the villi, the feto-placental vasculature also develops a complex branching structure in order to maximize exchange between the placental and maternal circulations. To understand the development of the placenta, we must translate functional information across spatial scales including the interaction between macro- and micro-scale haemodynamics and account for the effects of a dynamically and rapidly changing structure through the time course of pregnancy. Here, we present steps towards an anatomically based and multiscale approach to modelling the feto-placental circulation. We assess the effect of the location of cord insertion on feto-placental blood flow resistance and flow heterogeneity and show that, although cord insertion does not appear to directly influence feto-placental resistance, the heterogeneity of flow in the placenta is predicted to increase from a 19.4% coefficient of variation with central cord insertion to 23.3% when the cord is inserted 2 cm from the edge of the placenta. Model geometries with spheroidal and ellipsoidal shapes, but the same volume, showed no significant differences in flow resistance or heterogeneity, implying that normal asymmetry in shape does not affect placental efficiency. However, the size and number of small capillary vessels is predicted to have a large effect on feto-placental resistance and flow heterogeneity. Using this new model as an example, we highlight the importance of taking an integrated multi-disciplinary and multiscale approach to understand development of the placenta.
Keywords: computational model; feto–placental circulation; multiscale; placenta; pregnancy; vascular structure.
Figures




References
-
- Benirschke K, Kaufmann P. 1990. Pathology of the human placenta, pp. 180–243. New York, NY: Springer.
LinkOut - more resources
Full Text Sources
Other Literature Sources
