A multiscale model for the study of cardiac biomechanics in single-ventricle surgeries: a clinical case
- PMID: 25844151
- PMCID: PMC4342947
- DOI: 10.1098/rsfs.2014.0079
A multiscale model for the study of cardiac biomechanics in single-ventricle surgeries: a clinical case
Abstract
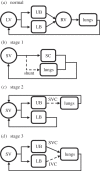
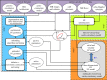
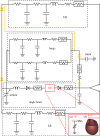
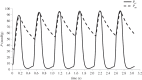
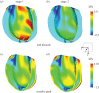
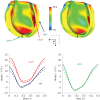
Complex congenital heart disease characterized by the underdevelopment of one ventricular chamber (single ventricle (SV) circulation) is normally treated with a three-stage surgical repair. This study aims at developing a multiscale computational framework able to couple a patient-specific three-dimensional finite-element model of the SV to a patient-specific lumped parameter (LP) model of the whole circulation, in a closed-loop fashion. A sequential approach was carried out: (i) cardiocirculatory parameters were estimated by using a fully LP model; (ii) ventricular material parameters and unloaded geometry were identified by means of the stand-alone, three-dimensional model of the SV; and (iii) the three-dimensional model of SV was coupled to the LP model of the circulation, thus closing the loop and creating a multiscale model. Once the patient-specific multiscale model was set using pre-operative clinical data, the virtual surgery was performed, and the post-operative conditions were simulated. This approach allows the analysis of local information on ventricular function as well as global parameters of the cardiovascular system. This methodology is generally applicable to patients suffering from SV disease for surgical planning at different stages of treatment. As an example, a clinical case from stage 1 to stage 2 is considered here.
Keywords: finite-element method; lumped parameter model; multiscale coupling; single ventricle heart; virtual surgery.
Figures



References
-
- Pekkan K, Whited B, Kanter K, Sharma S, De Zélicourt DA, Sundareswaran K, Frakes D, Rossignac J, Yoganathan AP. 2008. Patient-specific surgical planning and hemodynamic computational fluid dynamics optimization through free-form haptic anatomy editing tool (SURGEM). Med. Biol. Eng. Comput. 46, 1139–1152. (10.1007/s11517-008-0377-0) - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

