Lansoprazole Is Associated with Worsening Asthma Control in Children with the CYP2C19 Poor Metabolizer Phenotype
- PMID: 25844821
- PMCID: PMC4590024
- DOI: 10.1513/AnnalsATS.201408-391OC
Lansoprazole Is Associated with Worsening Asthma Control in Children with the CYP2C19 Poor Metabolizer Phenotype
Abstract
Rationale: Gastric acid blockade in children with asymptomatic acid reflux has not improved asthma control in published studies. There is substantial population variability regarding metabolism of and response to proton pump inhibitors based on metabolizer phenotype. How metabolizer phenotype affects asthma responses to acid blockage is not known.
Objectives: To determine how metabolizer phenotype based on genetic analysis of CYP2C19 affects asthma control among children treated with a proton pump inhibitor.
Methods: Asthma control as measured by the Asthma Control Questionnaire (ACQ) and other questionnaires from a 6-month clinical trial of lansoprazole in children with asthma was analyzed for associations with surrogates of lansoprazole exposure (based on treatment assignment and metabolizer phenotype). Groups included placebo-treated children; lansoprazole-treated extensive metabolizers (EMs); and lansoprazole-treated poor metabolizers (PMs). Metabolizer phenotypes were based on CYP2C19 haplotypes. Carriers of the CYP2C19*2, *3, *8, *9, or *10 allele were PMs; carriers of two wild-type alleles were extensive metabolizers (EMs).
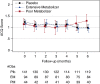
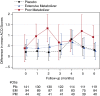
Measurements and main results: Asthma control through most of the treatment period was unaffected by lansoprazole exposure or metabolizer phenotype. At 6 months, PMs displayed significantly worsened asthma control compared with EMs (+0.16 vs. -0.13; P = 0.02) and placebo-treated children (+0.16 vs. -0.23; P < 0.01). Differences in asthma control were not associated with changes in gastroesophageal reflux symptoms. Recent upper respiratory infection worsened asthma control, and this upper respiratory infection effect may be more pronounced among lansoprazole-treated PMs.
Conclusions: Children with the PM phenotype developed worse asthma control after 6 months of lansoprazole treatment for poorly controlled asthma. Increased exposure to proton pump inhibitor may worsen asthma control by altering responses to respiratory infections. Clinical trial registered with www.clinicaltrials.gov (NCT00604851).
Keywords: child; lansoprazole; phenotype; polymorphism (genetics).
Figures
Comment in
-
Lansoprazole worsens asthma control in poor metabolizers: is nitric oxide involved?Ann Am Thorac Soc. 2015 Jul;12(7):1109-10. doi: 10.1513/AnnalsATS.201504-238LE. Ann Am Thorac Soc. 2015. PMID: 26203616 Free PMC article. No abstract available.
-
Reply: worsening asthma control in children taking lansoprazole: possible mechanisms.Ann Am Thorac Soc. 2015 Jul;12(7):1110-1. doi: 10.1513/AnnalsATS.201505-298LE. Ann Am Thorac Soc. 2015. PMID: 26203617 Free PMC article. No abstract available.
References
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, et al. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Long-term comparison of 3 controller regimens for mild–moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
-
- Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, Dozor AJ, Lima JJ, Mastronarde JG, Sockrider MM, et al. Writing Committee for the American Lung Association Asthma Clinical Research Centers. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307:373–381. - PMC - PubMed
-
- Blake K, Teague WG. Gastroesophageal reflux disease and childhood asthma. Curr Opin Pulm Med. 2013;19:24–29. - PubMed
-
- Furuta T, Ohashi K, Kosuge K, Zhao XJ, Takashima M, Kimura M, Nishimoto M, Hanai H, Kaneko E, Ishizaki T. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–561. - PubMed
-
- Shirai N, Furuta T, Xiao F, Kajimura M, Hanai H, Ohashi K, Ishizaki T. Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment Pharmacol Ther. 2002;16:837–846. - PubMed

