Particulate matter 2.5 induces autophagy via inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin kinase signaling pathway in human bronchial epithelial cells
- PMID: 25845384
- PMCID: PMC6918810
- DOI: 10.3892/mmr.2015.3577
Particulate matter 2.5 induces autophagy via inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin kinase signaling pathway in human bronchial epithelial cells
Abstract
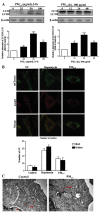
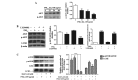
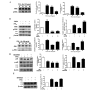
Particulate matter 2.5 (PM2.5) is a significant risk factor for asthma. A recent study revealed that autophagy was associated with asthma pathogenesis. However, the specific mechanisms underlying PM2.5-induced autophagy in asthma have remained elusive. In the present study, PM2.5-induced autophagy was evaluated in Beas-2B human bronchial epithelial cells and the potential molecular mechanisms were investigated. Using electron microscopy, immunofluorescence staining and immunoblot studies, it was confirmed that PM2.5 induced autophagy in Beas-2B cells as a result of PM2.5-mediated inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway in Beas-2B cells. LY294002, a PI3K inhibitor, reduced the accumulation of microtubule-associated protein 1 light chain 3 II and attenuated the effect of PM2.5. Phosphorylated (p-)p38, p-extracellular signal-regulated kinase and p-c-Jun N-terminal kinase were dephosphorylated following exposure to PM2.5. The roles of p53, reactive oxygen species scavenger tetramethylthiourea and autophagy inhibitor 3-methyladenine in PM2.5-induced autophagy in Beas-2B cells were also investigated. The results suggested that the PI3K/Akt/mTOR signaling pathway may be a key contributor to PM2.5-induced autophagy in Beas-2B cells. The results of the present study therefore provided an a insight into potential future clinical applications targeting these signaling pathways, for the prevention and/or treatment of PM2.5-induced lung diseases.
Figures


Similar articles
-
Atmospheric fine particulate matter exposure exacerbates atherosclerosis in apolipoprotein E knockout mice by inhibiting autophagy in macrophages via the PI3K/Akt/mTOR signaling pathway.Ecotoxicol Environ Saf. 2021 Jan 15;208:111440. doi: 10.1016/j.ecoenv.2020.111440. Epub 2020 Oct 8. Ecotoxicol Environ Saf. 2021. PMID: 33039868
-
Pingchuanning decoction attenuates airway inflammation by suppressing autophagy via phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in rat models of asthma.J Cell Biochem. 2019 Mar;120(3):3833-3844. doi: 10.1002/jcb.27665. Epub 2018 Sep 27. J Cell Biochem. 2019. PMID: 30260006
-
Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway.Chemosphere. 2017 Jan;167:444-453. doi: 10.1016/j.chemosphere.2016.10.024. Epub 2016 Oct 14. Chemosphere. 2017. PMID: 27750168
-
Natural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agents.Anticancer Agents Med Chem. 2013 Sep;13(7):1048-56. doi: 10.2174/18715206113139990130. Anticancer Agents Med Chem. 2013. PMID: 23293890 Review.
-
[PI3K/Akt/mTOR signal pathway in endocrine disrupting chemicals-induced apoptosis and autophagy of thyroid follicular cells].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021 Sep 20;39(9):717-720. doi: 10.3760/cma.j.cn121094-20201025-00510. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021. PMID: 34624962 Review. Chinese.
Cited by
-
IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy.J Cell Mol Med. 2020 Aug;24(15):8532-8544. doi: 10.1111/jcmm.15475. Epub 2020 Jul 9. J Cell Mol Med. 2020. Retraction in: J Cell Mol Med. 2024 Apr;28(7):e18222. doi: 10.1111/jcmm.18222. PMID: 32643865 Free PMC article. Retracted.
-
Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases.Oncol Lett. 2018 May;15(5):7506-7514. doi: 10.3892/ol.2018.8355. Epub 2018 Mar 26. Oncol Lett. 2018. PMID: 29725457 Free PMC article. Review.
-
Environmental Exposures and Asthma Development: Autophagy, Mitophagy, and Cellular Senescence.Front Immunol. 2019 Nov 29;10:2787. doi: 10.3389/fimmu.2019.02787. eCollection 2019. Front Immunol. 2019. PMID: 31849968 Free PMC article. Review.
-
Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks.Int J Mol Sci. 2019 Dec 24;21(1):136. doi: 10.3390/ijms21010136. Int J Mol Sci. 2019. PMID: 31878205 Free PMC article. Review.
-
Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract.Tuberc Respir Dis (Seoul). 2022 Jul;85(3):237-248. doi: 10.4046/trd.2021.0152. Epub 2022 Mar 23. Tuberc Respir Dis (Seoul). 2022. PMID: 35320665 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

