Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart
- PMID: 25846340
- PMCID: PMC5054830
- DOI: 10.1111/dom.12468
Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart
Abstract
Aims: To evaluate the pharmacokinetics and pharmacodynamics of faster-acting insulin aspart and insulin aspart in a randomized, single-centre, double-blind study.
Methods: Fifty-two patients with type 1 diabetes (mean age 40.3 years) received faster-acting insulin aspart, insulin aspart, or another faster aspart formulation (not selected for further development), each as a single 0.2 U/kg subcutaneous dose, under glucose-clamp conditions, in a three-way crossover design (3-12 days washout between dosing).
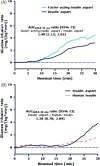
Results: Faster-acting insulin aspart had a faster onset of exposure compared with insulin aspart, shown by a 57% earlier onset of appearance [4.9 vs 11.2 min; ratio 0.43, 95% confidence interval (CI) 0.36; 0.51], a 35% earlier time to reach 50% maximum concentration (20.7 vs 31.6 min; ratio 0.65, 95% CI 0.59; 0.72) and a greater early exposure within 90 min after dosing. The greatest difference occurred during the first 15 min, when area under the serum insulin aspart curve was 4.5-fold greater with faster-acting insulin aspart than with insulin aspart. Both treatments had a similar time to maximum concentration, total exposure and maximum concentration. Faster-acting insulin aspart had a significantly greater glucose-lowering effect within 90 min after dosing [largest difference: area under the curve for the glucose infusion rate (AUC(GIR), 0-30 min) ratio 1.48, 95% CI 1.13; 2.02] and 17% earlier time to reach 50% maximum glucose infusion rate (38.3 vs 46.1 min; ratio 0.83, 95% CI 0.73; 0.94). The primary endpoint (AUC(GIR, 0-2 h)) was 10% greater for faster-acting insulin aspart, but did not reach statistical significance (ratio 1.10, 95% CI 1.00; 1.22). Both treatments had similar total and maximum glucose-lowering effects, indicating similar overall potency.
Conclusions: Faster-acting insulin aspart was found to have earlier onset and higher early exposure than insulin aspart, and a greater early glucose-lowering effect, with similar potency.
Trial registration: ClinicalTrials.gov NCT01618188.
Keywords: faster-acting insulin aspart; pharmacodynamics; pharmacokinetics; postprandial glucose; type 1 diabetes.
© 2015 John Wiley & Sons Ltd.
Figures


References
-
- Woerle HJ, Neumann C, Zschau S et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007; 77: 280–285. - PubMed
-
- International Diabetes Federation Guideline Development Group . Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract 2014; 103: 256–268. - PubMed
-
- Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time‐dependent effects of glucose. Diabetes 2002; 51(Suppl. 1): S53–59. - PubMed
-
- Bruce DG, Chisholm DJ, Storlien LH, Kraegen EW. Physiological importance of deficiency in early prandial insulin secretion in non‐insulin‐dependent diabetes. Diabetes 1988; 37: 736–744. - PubMed
-
- Del Prato S, Tiengo A. The importance of first‐phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev 2001; 17: 164–174. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

