Sialylation regulates brain structure and function
- PMID: 25846372
- PMCID: PMC4478807
- DOI: 10.1096/fj.15-270983
Sialylation regulates brain structure and function
Abstract
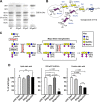
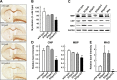
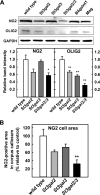
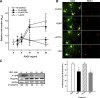
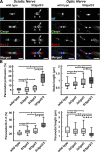
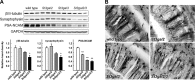
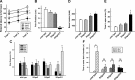
Every cell expresses a molecularly diverse surface glycan coat (glycocalyx) comprising its interface with its cellular environment. In vertebrates, the terminal sugars of the glycocalyx are often sialic acids, 9-carbon backbone anionic sugars implicated in intermolecular and intercellular interactions. The vertebrate brain is particularly enriched in sialic acid-containing glycolipids termed gangliosides. Human congenital disorders of ganglioside biosynthesis result in paraplegia, epilepsy, and intellectual disability. To better understand sialoglycan functions in the nervous system, we studied brain anatomy, histology, biochemistry, and behavior in mice with engineered mutations in St3gal2 and St3gal3, sialyltransferase genes responsible for terminal sialylation of gangliosides and some glycoproteins. St3gal2/3 double-null mice displayed dysmyelination marked by a 40% reduction in major myelin proteins, 30% fewer myelinated axons, a 33% decrease in myelin thickness, and molecular disruptions at nodes of Ranvier. In part, these changes may be due to dysregulation of ganglioside-mediated oligodendroglial precursor cell proliferation. Neuronal markers were also reduced up to 40%, and hippocampal neurons had smaller dendritic arbors. Young adult St3gal2/3 double-null mice displayed impaired motor coordination, disturbed gait, and profound cognitive disability. Comparisons among sialyltransferase mutant mice provide insights into the functional roles of brain gangliosides and sialoglycoproteins consistent with related human congenital disorders.
Keywords: animal models; behavior; gangliosides; myelin; oligodendrocyte precursor cells.
© FASEB.
Figures


Similar articles
-
Biosynthesis of the major brain gangliosides GD1a and GT1b.Glycobiology. 2012 Oct;22(10):1289-301. doi: 10.1093/glycob/cws103. Epub 2012 Jun 26. Glycobiology. 2012. PMID: 22735313 Free PMC article.
-
Sialyltransferase Mutations Alter the Expression of Calcium-Binding Interneurons in Mice Neocortex, Hippocampus and Striatum.Int J Mol Sci. 2023 Dec 7;24(24):17218. doi: 10.3390/ijms242417218. Int J Mol Sci. 2023. PMID: 38139047 Free PMC article.
-
Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration.Physiol Rev. 2014 Apr;94(2):461-518. doi: 10.1152/physrev.00033.2013. Physiol Rev. 2014. PMID: 24692354 Free PMC article. Review.
-
In vitro analysis of glial cell function in ganglioside-deficient mice.J Neurosci Res. 2009 Aug 15;87(11):2467-83. doi: 10.1002/jnr.22085. J Neurosci Res. 2009. PMID: 19382235
-
The vertebrate sialylation machinery: structure-function and molecular evolution of GT-29 sialyltransferases.Glycoconj J. 2023 Aug;40(4):473-492. doi: 10.1007/s10719-023-10123-w. Epub 2023 May 29. Glycoconj J. 2023. PMID: 37247156 Free PMC article. Review.
Cited by
-
Associations between attention-deficit/hyperactivity disorder and autoimmune diseases are modified by sex: a population-based cross-sectional study.Eur Child Adolesc Psychiatry. 2018 May;27(5):663-675. doi: 10.1007/s00787-017-1056-1. Epub 2017 Oct 5. Eur Child Adolesc Psychiatry. 2018. PMID: 28983730 Free PMC article.
-
Free sialic acid storage disorder: Progress and promise.Neurosci Lett. 2021 Jun 11;755:135896. doi: 10.1016/j.neulet.2021.135896. Epub 2021 Apr 20. Neurosci Lett. 2021. PMID: 33862140 Free PMC article. Review.
-
Activated microglia desialylate their surface, stimulating complement receptor 3-mediated phagocytosis of neurons.Glia. 2020 May;68(5):989-998. doi: 10.1002/glia.23757. Epub 2019 Nov 27. Glia. 2020. PMID: 31774586 Free PMC article.
-
RREB1 regulates neuronal proteostasis and the microtubule network.Sci Adv. 2024 Jan 12;10(2):eadh3929. doi: 10.1126/sciadv.adh3929. Epub 2024 Jan 10. Sci Adv. 2024. PMID: 38198538 Free PMC article.
-
Increasing brain N-acetylneuraminic acid alleviates hydrocephalus-induced neurological deficits.CNS Neurosci Ther. 2023 Nov;29(11):3183-3198. doi: 10.1111/cns.14253. Epub 2023 May 24. CNS Neurosci Ther. 2023. PMID: 37222223 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

