A library of synthetic transcription activator-like effector-activated promoters for coordinated orthogonal gene expression in plants
- PMID: 25846505
- PMCID: PMC4691316
- DOI: 10.1111/tpj.12843
A library of synthetic transcription activator-like effector-activated promoters for coordinated orthogonal gene expression in plants
Abstract
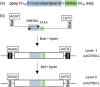
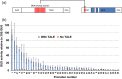
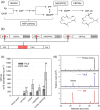
A library of synthetic promoters containing the binding site of a single designer transcription activator-like effector (dTALE) was constructed. The promoters contain a constant sequence, consisting of an 18-base long dTALE-binding site and a TATA box, flanked by degenerate sequences of 49 bases downstream and 19 bases upstream. Forty-three of these promoters were sequenced and tested in transient assays in Nicotiana benthamiana using a GUS reporter gene. The strength of expression of the promoters ranged from around 5% to almost 100% of the viral 35S promoter activity. We then demonstrated the utility of these promoters for metabolic engineering by transiently expressing three genes for the production of a plant diterpenoid in N. benthamiana. The simplicity of the promoter structure shows great promise for the development of genetic circuits, with wide potential applications in plant synthetic biology and metabolic engineering.
Keywords: Nicotiana benthamiana; metabolic engineering; orthogonality; plants; synthetic biology; synthetic promoters; technical advance; terpenoid; transcription activator-like effectors; transient assays.
© 2015 The Authors The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Figures


References
-
- Blazeck J, Alper HS. Promoter engineering: recent advances in controlling transcription at the most fundamental level. Biotechnol. J. 2013;8:46–58. - PubMed
-
- Boch J, Bonas U. Xanthomonas AvrBs3 Family-Type III effectors: discovery and function. Annu. Rev. Phytopathol. 2010;48:419–436. - PubMed
-
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

