Amygdala pain mechanisms
- PMID: 25846623
- PMCID: PMC4701385
- DOI: 10.1007/978-3-662-46450-2_13
Amygdala pain mechanisms
Abstract
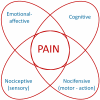
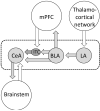
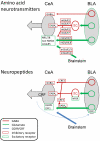
A limbic brain area, the amygdala plays a key role in emotional responses and affective states and disorders such as learned fear, anxiety, and depression. The amygdala has also emerged as an important brain center for the emotional-affective dimension of pain and for pain modulation. Hyperactivity in the laterocapsular division of the central nucleus of the amygdala (CeLC, also termed the "nociceptive amygdala") accounts for pain-related emotional responses and anxiety-like behavior. Abnormally enhanced output from the CeLC is the consequence of an imbalance between excitatory and inhibitory mechanisms. Impaired inhibitory control mediated by a cluster of GABAergic interneurons in the intercalated cell masses (ITC) allows the development of glutamate- and neuropeptide-driven synaptic plasticity of excitatory inputs from the brainstem (parabrachial area) and from the lateral-basolateral amygdala network (LA-BLA, site of integration of polymodal sensory information). BLA hyperactivity also generates abnormally enhanced feedforward inhibition of principal cells in the medial prefrontal cortex (mPFC), a limbic cortical area that is strongly interconnected with the amygdala. Pain-related mPFC deactivation results in cognitive deficits and failure to engage cortically driven ITC-mediated inhibitory control of amygdala processing. Impaired cortical control allows the uncontrolled persistence of amygdala pain mechanisms.
Figures

Similar articles
-
Amygdala Plasticity and Pain.Pain Res Manag. 2017;2017:8296501. doi: 10.1155/2017/8296501. Epub 2017 Dec 10. Pain Res Manag. 2017. PMID: 29302197 Free PMC article. Review.
-
Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1.Mol Pain. 2010 Dec 16;6:93. doi: 10.1186/1744-8069-6-93. Mol Pain. 2010. PMID: 21162731 Free PMC article.
-
Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation.J Neurosci. 2010 Apr 14;30(15):5451-64. doi: 10.1523/JNEUROSCI.0225-10.2010. J Neurosci. 2010. PMID: 20392966 Free PMC article.
-
Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors.J Neurophysiol. 2013 Oct;110(8):1765-81. doi: 10.1152/jn.00874.2012. Epub 2013 Jul 24. J Neurophysiol. 2013. PMID: 23883857 Free PMC article.
-
Amygdala, neuropeptides, and chronic pain-related affective behaviors.Neuropharmacology. 2020 Jun 15;170:108052. doi: 10.1016/j.neuropharm.2020.108052. Epub 2020 Mar 15. Neuropharmacology. 2020. PMID: 32188569 Free PMC article. Review.
Cited by
-
Infant pain vs. pain with parental suppression: Immediate and enduring impact on brain, pain and affect.PLoS One. 2023 Nov 16;18(11):e0290871. doi: 10.1371/journal.pone.0290871. eCollection 2023. PLoS One. 2023. PMID: 37972112 Free PMC article.
-
Anxiety Specific Response and Contribution of Active Hippocampal Neural Stem Cells to Chronic Pain Through Wnt/β-Catenin Signaling in Mice.Front Mol Neurosci. 2018 Aug 24;11:296. doi: 10.3389/fnmol.2018.00296. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30197587 Free PMC article.
-
A psychometric evaluation of the Italian short version of the Fear of Pain Questionnaire-III: Psychometric properties, measurement invariance across gender, convergent, and discriminant validity.Front Psychol. 2023 Jan 11;13:1087055. doi: 10.3389/fpsyg.2022.1087055. eCollection 2022. Front Psychol. 2023. PMID: 36726497 Free PMC article.
-
Input-dependent synaptic suppression by pregabalin in the central amygdala in male mice with inflammatory pain.Neurobiol Pain. 2021 Nov 18;10:100078. doi: 10.1016/j.ynpai.2021.100078. eCollection 2021 Aug-Dec. Neurobiol Pain. 2021. PMID: 34877437 Free PMC article.
-
Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain.Pain. 2019 Apr;160(4):824-832. doi: 10.1097/j.pain.0000000000001458. Pain. 2019. PMID: 30681985 Free PMC article.
References
-
- Ansah OB, Bourbia N, Goncalves L, Almeida A, Pertovaara A. Influence of amygdaloid glutamatergic receptors on sensory and emotional pain-related behavior in the neuropathic rat. Behav Brain Res. 2010;209:174–178. - PubMed
-
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The Corticotropin-Releasing Factor (CRF)-system and monoaminergic afferents in the central amygdala: Investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

