Beta-interferon exposure and onset of secondary progressive multiple sclerosis
- PMID: 25846809
- PMCID: PMC5008210
- DOI: 10.1111/ene.12698
Beta-interferon exposure and onset of secondary progressive multiple sclerosis
Abstract
Background and purpose: Beta-interferons (IFNβ) are the most widely prescribed drugs for patients with multiple sclerosis (MS). However, whether or not treatment with IFNβ can delay secondary progressive MS (SPMS) onset remains unknown. Our aim was to examine the association between IFNβ exposure and SPMS onset in patients with relapsing-remitting MS (RRMS).
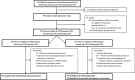
Methods: A retrospective cohort study using British Columbia (Canada) population-based clinical and health administrative data (1985-2008) was conducted. RRMS patients treated with IFNβ (n = 794) were compared with untreated contemporary (n = 933) and historical (n = 837) controls. Cohort entry was the first clinic visit during which patients became eligible for IFNβ treatment (baseline). The outcome was time from baseline to SPMS onset. Cox regression models with IFNβ as a time-dependent exposure were adjusted for sex, and baseline age, disease duration, disability, *socioeconomic status and *comorbidities (*available for the contemporary cohorts only). Additional analyses included propensity score adjustment.
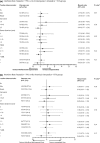
Results: The median follow-up for the IFNβ-treated, untreated contemporary and historical controls were 5.7, 3.7 and 7.3 years, and the proportions of patients reaching SPMS were 9.2%, 11.8% and 32.9%, respectively. After adjustment for confounders, IFNβ exposure was not associated with the risk of reaching SPMS when either the contemporary or the historical untreated cohorts were considered (hazard ratio 1.07; 95% confidence interval 0.93-1.48, and hazard ratio 1.04; 95% confidence interval 0.74-1.46, respectively). Further adjustments and the propensity score yielded results consistent with the main analysis.
Conclusions: Amongst patients with RRMS, use of IFNβ was not associated with a delayed onset of SPMS.
Keywords: beta-interferon; cohort study; multiple sclerosis; progression.
© 2015 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Figures


References
-
- The Multiple Sclerosis International Federation . An Atlas of MS. http://www.atlasofms.org/ (accessed 19/09/2013).
-
- Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010; 74: 2004–2015. - PubMed
-
- Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary‐progressive multiple sclerosis. Mult Scler 2008; 14: 314–324. - PubMed
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

