Smoking Status Confirmation by Proxy: Validation in a Smoking Cessation Trial
- PMID: 25847290
- PMCID: PMC4707186
- DOI: 10.1093/ntr/ntv073
Smoking Status Confirmation by Proxy: Validation in a Smoking Cessation Trial
Abstract
Introduction: Biochemical confirmation (BC) of self-report is the gold standard of evidence for abstinence in smoking cessation research, but difficulty in obtaining samples may bias estimates of quit rates. Proxy confirmation (PC) has not been validated in cessation trials. We assessed the feasibility and validity of PC in a cessation trial for hospitalized smokers.
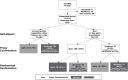
Methods: We enrolled 402 daily cigarette smokers during a hospital admission. At enrollment, participants provided demographics, smoking history, and named proxies to confirm their smoking status at follow-up. Participants provided self-reported (SR) 7-day tobacco abstinence by telephone at 6 months post-discharge. SR quitters were asked to mail a saliva sample for BC. Incentives were offered for survey completion ($20) and returned samples ($50). We called proxies for all those with SR to obtain PC. Quit rates were calculated with missing data indicating smoking. We assessed associations of nonresponse with baseline characteristics using chi-squared tests and logistic regression. We calculated the sensitivity and specificity of PC in detecting smokers as determined by BC.
Results: All patients named at least one proxy. Response rates were 82% for SR, 84% for PC, and 69% for BC. Observed participant characteristics were unrelated to provision of sample for BC. Estimated quit rates were 35% for SR, 27% for SR + PC, 21% for SR + BC and 27% for SR + BC or PC. Sensitivity of PC was not higher than SR (73% vs. 77%); specificity was lower (84% vs. 100%).
Conclusion: PC was feasible but not superior to self-report in a cessation trial.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. 10.1080/14622200210123581. - PubMed
-
- McLaughlin JK, Dietz MS, Mehl ES, Blot WJ. Reliability of surrogate information on cigarette smoking by type of informant. Am J Epidemiol. 1987;126(1):144–146. - PubMed
-
- Hyland A, Cummings KM, Lynn WR, Corle D, Giffen CA. Effect of proxy-reported smoking status on population estimates of smoking prevalence. Am J Epidemiol. 1997;145(8):746–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials