Functional mechanisms of neurotransmitter transporters regulated by lipid-protein interactions of their terminal loops
- PMID: 25847498
- PMCID: PMC4501894
- DOI: 10.1016/j.bbamem.2015.03.025
Functional mechanisms of neurotransmitter transporters regulated by lipid-protein interactions of their terminal loops
Abstract
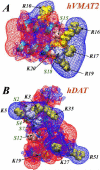
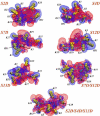
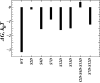
The physiological functions of neurotransmitter:sodium symporters (NSS) in reuptake of neurotransmitters from the synapse into the presynaptic nerve have been shown to be complemented by their involvement, together with non-plasma membrane neurotransmitter transporters, in the reverse transport of substrate (efflux) in response to psychostimulants. Recent experimental evidence implicates highly anionic phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids in such functions of the serotonin (SERT) and dopamine (DAT) transporters. Thus, for both SERT and DAT, neurotransmitter efflux has been shown to be strongly regulated by the presence of PIP(2) lipids in the plasma membrane, and the electrostatic interaction of the N-terminal region of DAT with the negatively charged PIP(2) lipids. We examine the experimentally established phenotypes in a structural context obtained from computational modeling based on recent crystallographic data. The results are shown to set the stage for a mechanistic understanding of physiological actions of neurotransmitter transporters in the NSS family of membrane proteins. This article is part of a Special Issue entitled: Lipid-protein interactions.
Keywords: Amphetamine-induced efflux; Cell signaling and phosphorylation; Continuum mean-field theory; Electrostatic interactions; Lipid segregation in the membrane; Membrane composition and PIP(2) lipids; Molecular dynamics; Molecular dynamics simulations; Psychostimulant drugs of abuse.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Computational modeling of the N-terminus of the human dopamine transporter and its interaction with PIP2 -containing membranes.Proteins. 2015 May;83(5):952-69. doi: 10.1002/prot.24792. Epub 2015 Mar 25. Proteins. 2015. PMID: 25739722 Free PMC article.
-
Templates and models of monoamine transporter proteins.Curr Med Chem. 2011;18(30):4651-8. doi: 10.2174/092986711797379203. Curr Med Chem. 2011. PMID: 21864275
-
How structural elements evolving from bacterial to human SLC6 transporters enabled new functional properties.BMC Biol. 2018 Mar 14;16(1):31. doi: 10.1186/s12915-018-0495-6. BMC Biol. 2018. PMID: 29540172 Free PMC article.
-
Neurotransmitter transporters in schistosomes: structure, function and prospects for drug discovery.Parasitol Int. 2013 Dec;62(6):629-38. doi: 10.1016/j.parint.2013.06.003. Epub 2013 Jun 22. Parasitol Int. 2013. PMID: 23800409 Review.
-
Structural insights into functional lipid-protein interactions in secondary transporters.Biochim Biophys Acta. 2015 Mar;1850(3):476-87. doi: 10.1016/j.bbagen.2014.05.010. Epub 2014 May 20. Biochim Biophys Acta. 2015. PMID: 24859688 Review.
Cited by
-
The tether function of the anoctamins.Cell Calcium. 2024 Jul;121:102875. doi: 10.1016/j.ceca.2024.102875. Epub 2024 Apr 20. Cell Calcium. 2024. PMID: 38701708 Free PMC article. Review.
-
Dopamine transporter membrane mobility is bidirectionally regulated by phosphorylation and palmitoylation.Curr Res Physiol. 2023 Sep 29;6:100106. doi: 10.1016/j.crphys.2023.100106. eCollection 2023. Curr Res Physiol. 2023. PMID: 38107792 Free PMC article. Review.
-
Insights into the mechanism and pharmacology of neurotransmitter sodium symporters.Curr Opin Struct Biol. 2019 Feb;54:161-170. doi: 10.1016/j.sbi.2019.03.011. Epub 2019 Mar 25. Curr Opin Struct Biol. 2019. PMID: 30921707 Free PMC article. Review.
-
Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action.Adv Pharmacol. 2018;82:205-234. doi: 10.1016/bs.apha.2017.09.002. Epub 2017 Oct 25. Adv Pharmacol. 2018. PMID: 29413521 Free PMC article. Review.
-
Regulation of monoamine transporters and receptors by lipid microdomains: implications for depression.Neuropsychopharmacology. 2018 Oct;43(11):2165-2179. doi: 10.1038/s41386-018-0133-6. Epub 2018 Jun 28. Neuropsychopharmacology. 2018. PMID: 30022062 Free PMC article. Review.
References
-
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. - PubMed
-
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. - PubMed
-
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

