TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer
- PMID: 25847936
- PMCID: PMC4451173
- DOI: 10.1200/JCO.2014.57.6660
TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer
Abstract
Purpose: The identification of patients with metastatic triple-negative breast cancer (mTNBC) who are expected to benefit from platinum-based chemotherapy is of interest. We conducted a single-arm phase II clinical trial of single-agent platinum for mTNBC with biomarker correlates.
Patients and methods: Patients with mTNBC received first- or second-line cisplatin (75 mg/m(2)) or carboplatin (area under the concentration-time curve 6) by physician's choice once every 3 weeks. Coprimary end points were objective response rate (RR) and response prediction by p63/p73 gene expression. Secondary and exploratory end points included toxicity assessment, RR in cisplatin versus carboplatin, and RR in molecularly defined subgroups, including BRCA1/2 mutation carriers.
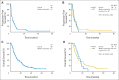
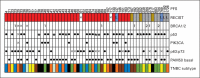
Results: Patients (N = 86; 69 as first-line therapy) received cisplatin (n = 43) or carboplatin (n = 43). RR was 25.6% (95% CI, 16.8% to 36%) and was numerically higher with cisplatin (32.6%) than with carboplatin (18.7%). RR was 54.5% in patients with germline BRCA1/2 mutations (n = 11). In patients without BRCA1/2 mutations (n = 66), exploratory analyses showed that a BRCA-like genomic instability signature (n = 32) discriminated responding and nonresponding tumors (mean homologous recombination deficiency-loss of heterozygosity/homologous recombination deficiency-large-scale state transitions [HRD-LOH/HRD-LST] scores were 12.68 and 5.11, respectively), whereas predefined analysis by p63/p73 expression status (n = 61), p53 and PIK3CA mutation status (n = 53), or PAM50 gene expression subtype (n = 55) did not. Five of the six long-term responders alive at a median of 4.5 years lacked germline BRCA1/2 mutations, and two of them had increased tumor HRD-LOH/HRD-LST scores.
Conclusion: Platinum agents are active in mTNBC, especially in patients with germline BRCA1/2 mutations. A measure of tumor DNA repair function may identify patients without mutations who could benefit from platinum therapy agents. Prospective controlled confirmatory trials are warranted.
Trial registration: ClinicalTrials.gov NCT00483223.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Challenges in the Use of DNA Repair Deficiency As a Biomarker in Breast Cancer.J Clin Oncol. 2015 Jun 10;33(17):1867-9. doi: 10.1200/JCO.2014.60.5501. Epub 2015 Apr 27. J Clin Oncol. 2015. PMID: 25918281 No abstract available.
References
-
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. - PubMed
-
- Hudis CA, Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16:1–11. - PubMed
-
- Criscitiello C, Azim HA, Jr, Schouten PC, et al. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23:vi13–vi18. - PubMed
-
- Turner NC, Reis-Filho JS. Tackling the diversity of triple-negative breast cancer. Clin Cancer Res. 2013;19:6380–6388. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

