AMA1-deficient Toxoplasma gondii parasites transiently colonize mice and trigger an innate immune response that leads to long-lasting protective immunity
- PMID: 25847964
- PMCID: PMC4432741
- DOI: 10.1128/IAI.02606-14
AMA1-deficient Toxoplasma gondii parasites transiently colonize mice and trigger an innate immune response that leads to long-lasting protective immunity
Abstract
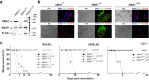
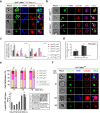
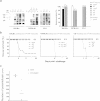
The apical membrane antigen 1 (AMA1) protein was believed to be essential for the perpetuation of two Apicomplexa parasite genera, Plasmodium and Toxoplasma, until we genetically engineered viable parasites lacking AMA1. The reduction in invasiveness of the Toxoplasma gondii RH-AMA1 knockout (RH-AMA1(KO)) tachyzoite population, in vitro, raised key questions about the outcome associated with these tachyzoites once inoculated in the peritoneal cavity of mice. In this study, we used AMNIS technology to simultaneously quantify and image the parasitic process driven by AMA1(KO) tachyzoites. We report their ability to colonize and multiply in mesothelial cells and in both resident and recruited leukocytes. While the RH-AMA1(KO) population amplification is rapidly lethal in immunocompromised mice, it is controlled in immunocompetent hosts, where immune cells in combination sense parasites and secrete proinflammatory cytokines. This innate response further leads to a long-lasting status immunoprotective against a secondary challenge by high inocula of the homologous type I or a distinct type II T. gondii genotypes. While AMA1 is definitively not an essential protein for tachyzoite entry and multiplication in host cells, it clearly assists the expansion of parasite population in vivo.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP, Sibley LD. 2012. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A 109:5844–5849. doi:10.1073/pnas.1203190109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

