Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant
- PMID: 25847977
- PMCID: PMC4565994
- DOI: 10.1093/cid/civ272
Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant
Erratum in
-
Campbell AP et al (Clin Infect Dis 2015; 61: 192-202).Clin Infect Dis. 2015 Nov 15;61(10):1635. doi: 10.1093/cid/civ800. Epub 2015 Sep 24. Clin Infect Dis. 2015. PMID: 26405149 Free PMC article. No abstract available.
Abstract
Background: The management of respiratory virus infections prior to hematopoietic cell transplant (HCT) is difficult. We examined whether respiratory virus detection before HCT influenced the requirement for bronchoscopy, hospitalization, and overall survival following HCT.
Methods: Pre-HCT and weekly post-HCT nasal washes were collected through day 100 from patients with and without symptoms. Samples were tested by multiplex polymerase chain reaction for respiratory syncytial virus, parainfluenza viruses 1-4, influenza A and B, human metapneumovirus, adenovirus, and human rhinoviruses, coronaviruses, and bocavirus.
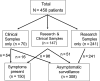
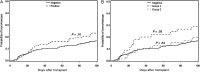
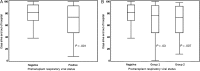
Results: Of 458 patients, 116 (25%) had respiratory viruses detected pre-HCT. Overall, patients with viruses detected pre-HCT had fewer days alive and out of the hospital and lower survival at day 100 (adjusted hazard ratio [aHR], 2.4; 95% confidence interval [CI], 1.3-4.5; P = .007) than patients with negative samples; this risk was also present with rhinovirus alone (aHR for mortality, 2.6; 95% CI, 1.2-5.5; P = .01). No difference in bronchoscopy incidence was seen in patients with and without respiratory viruses (aHR, 1.3; 95% CI, .8-2.0; P = .32). In symptomatic patients, those with respiratory viruses detected had increased overall mortality compared with patients without viruses detected (unadjusted HR, 3.5; 95% CI, 1.0-12.1; P = .05); among asymptomatic patients, detection of respiratory viruses was not associated with increased mortality.
Conclusions: These data support routine testing for respiratory viruses among symptomatic patients before HCT, and delay of transplant with virus detection when feasible, even for detection of rhinovirus alone. Further study is needed to address whether asymptomatic patients should undergo screening for respiratory virus detection before HCT.
Keywords: hematopoietic cell transplant; pneumonia; respiratory virus infection.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. - PMC - PubMed
-
- Peck AJ, Corey L, Boeckh M. Pretransplantation respiratory syncytial virus infection: impact of a strategy to delay transplantation. Clin Infect Dis 2004; 39:673–80. - PubMed
-
- Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant 1989; 4:35–40. - PubMed
-
- Bredius RG, Templeton KE, Scheltinga SA, Claas EC, Kroes AC, Vossen JM. Prospective study of respiratory viral infections in pediatric hemopoietic stem cell transplantation patients. Pediatr Infect Dis J 2004; 23:518–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA18029/CA/NCI NIH HHS/United States
- R01 HL081595/HL/NHLBI NIH HHS/United States
- HL93294/HL/NHLBI NIH HHS/United States
- K23 HL091059/HL/NHLBI NIH HHS/United States
- L40AI071572/AI/NIAID NIH HHS/United States
- CA15704/CA/NCI NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- HL081595/HL/NHLBI NIH HHS/United States
- K23HL091059/HL/NHLBI NIH HHS/United States
- K24 HL093294/HL/NHLBI NIH HHS/United States
- L40 AI071572/AI/NIAID NIH HHS/United States
- ULI RR025014/RR/NCRR NIH HHS/United States
- P01 CA018029/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

