Stress sigma factor RpoS degradation and translation are sensitive to the state of central metabolism
- PMID: 25847996
- PMCID: PMC4413282
- DOI: 10.1073/pnas.1504639112
Stress sigma factor RpoS degradation and translation are sensitive to the state of central metabolism
Abstract
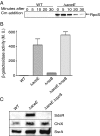
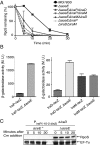
RpoS, the stationary phase/stress sigma factor of Escherichia coli, regulates a large cohort of genes important for the cell to deal with suboptimal conditions. Its level increases quickly in the cell in response to many stresses and returns to low levels when growth resumes. Increased RpoS results from increased translation and decreased RpoS degradation. Translation is positively regulated by small RNAs (sRNAs). Protein stability is positively regulated by anti-adaptors, which prevent the RssB adaptor-mediated degradation of RpoS by the ClpXP protease. Inactivation of aceE, a subunit of pyruvate dehydrogenase (PDH), was found to increase levels of RpoS by affecting both translation and protein degradation. The stabilization of RpoS in aceE mutants is dependent on increased transcription and translation of IraP and IraD, two known anti-adaptors. The aceE mutation also leads to a significant increase in rpoS translation. The sRNAs known to positively regulate RpoS are not responsible for the increased translation; sequences around the start codon are sufficient for the induction of translation. PDH synthesizes acetyl-CoA; acetate supplementation allows the cell to synthesize acetyl-CoA by an alternative, less favored pathway, in part dependent upon RpoS. Acetate addition suppressed the effects of the aceE mutant on induction of the anti-adaptors, RpoS stabilization, and rpoS translation. Thus, the bacterial cell responds to lowered levels of acetyl-CoA by inducing RpoS, allowing reprogramming of E. coli metabolism.
Keywords: ClpXP; RpoS; RssB; acetyl CoA; pyruvate dehydrogenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol Microbiol. 2008;68(2):298–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

