Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus
- PMID: 25848010
- PMCID: PMC4413319
- DOI: 10.1073/pnas.1424435112
Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus
Abstract
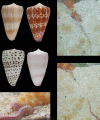
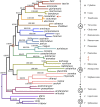
Prey shifts in carnivorous predators are events that can initiate the accelerated generation of new biodiversity. However, it is seldom possible to reconstruct how the change in prey preference occurred. Here we describe an evolutionary "smoking gun" that illuminates the transition from worm hunting to fish hunting among marine cone snails, resulting in the adaptive radiation of fish-hunting lineages comprising ∼100 piscivorous Conus species. This smoking gun is δ-conotoxin TsVIA, a peptide from the venom of Conus tessulatus that delays inactivation of vertebrate voltage-gated sodium channels. C. tessulatus is a species in a worm-hunting clade, which is phylogenetically closely related to the fish-hunting cone snail specialists. The discovery of a δ-conotoxin that potently acts on vertebrate sodium channels in the venom of a worm-hunting cone snail suggests that a closely related ancestral toxin enabled the transition from worm hunting to fish hunting, as δ-conotoxins are highly conserved among fish hunters and critical to their mechanism of prey capture; this peptide, δ-conotoxin TsVIA, has striking sequence similarity to these δ-conotoxins from piscivorous cone snail venoms. Calcium-imaging studies on dissociated dorsal root ganglion (DRG) neurons revealed the peptide's putative molecular target (voltage-gated sodium channels) and mechanism of action (inhibition of channel inactivation). The results were confirmed by electrophysiology. This work demonstrates how elucidating the specific interactions between toxins and receptors from phylogenetically well-defined lineages can uncover molecular mechanisms that underlie significant evolutionary transitions.
Keywords: cone snails; conotoxin; evolution; prey preference.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Michener CD. The Bees of the World. Johns Hopkins Univ Press; Baltimore: 2000.
-
- Jollès J, et al. Episodic evolution in the stomach lysozymes of ruminants. J Mol Evol. 1989;28(6):528–535. - PubMed
-
- Jollès J, et al. Amino acid sequences of stomach and nonstomach lysozymes of ruminants. J Mol Evol. 1990;30(4):370–382. - PubMed
-
- Kornegay JR. Molecular genetics and evolution of stomach and nonstomach lysozymes in the hoatzin. J Mol Evol. 1996;42(6):676–684. - PubMed
-
- Kornegay JR, Schilling JW, Wilson AC. Molecular adaptation of a leaf-eating bird: Stomach lysozyme of the hoatzin. Mol Biol Evol. 1994;11(6):921–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

