Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria
- PMID: 25848019
- PMCID: PMC4547279
- DOI: 10.1073/pnas.1421379112
Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria
Abstract
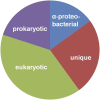
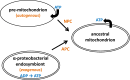
Comparative studies of the mitochondrial proteome have identified a conserved core of proteins descended from the α-proteobacterial endosymbiont that gave rise to the mitochondrion and was the source of the mitochondrial genome in contemporary eukaryotes. A surprising result of phylogenetic analyses is the relatively small proportion (10-20%) of the mitochondrial proteome displaying a clear α-proteobacterial ancestry. A large fraction of mitochondrial proteins typically has detectable homologs only in other eukaryotes and is presumed to represent proteins that emerged specifically within eukaryotes. A further significant fraction of the mitochondrial proteome consists of proteins with homologs in prokaryotes, but without a robust phylogenetic signal affiliating them with specific prokaryotic lineages. The presumptive evolutionary source of these proteins is quite different in contending models of mitochondrial origin.
Keywords: endosymbiont; mitochondria; phagotrophy; proteome; syntrophy.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14(3):255–274. - PubMed
-
- Margulis L. Origin of Eukaryotic Cells. Yale Univ Press; New Haven, CT: 1970.
-
- O’Malley MA. The first eukaryote cell: An unfinished history of contestation. Stud Hist Philos Biol Biomed Sci. 2010;41(3):212–224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

