Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases
- PMID: 25848049
- PMCID: PMC4413331
- DOI: 10.1073/pnas.1423536112
Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases
Retraction in
-
Retraction for Germain et al., Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases.Proc Natl Acad Sci U S A. 2019 May 28;116(22):11077. doi: 10.1073/pnas.1906160116. Epub 2019 May 20. Proc Natl Acad Sci U S A. 2019. PMID: 31110010 Free PMC article. No abstract available.
Abstract
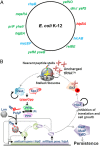
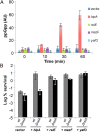
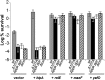
The model organism Escherichia coli codes for at least 11 type II toxin-antitoxin (TA) modules, all implicated in bacterial persistence (multidrug tolerance). Ten of these encode messenger RNA endonucleases (mRNases) inhibiting translation by catalytic degradation of mRNA, and the 11th module, hipBA, encodes HipA (high persister protein A) kinase, which inhibits glutamyl tRNA synthetase (GltX). In turn, inhibition of GltX inhibits translation and induces the stringent response and persistence. Previously, we presented strong support for a model proposing (p)ppGpp (guanosine tetra and penta-phosphate) as the master regulator of persistence. Stochastic variation of [(p)ppGpp] in single cells induced TA-encoded mRNases via a pathway involving polyphosphate and Lon protease. Polyphosphate activated Lon to degrade all known type II antitoxins of E. coli. In turn, the activated mRNases induced persistence and multidrug tolerance. However, even though it was known that activation of HipA stimulated (p)ppGpp synthesis, our model did not explain how hipBA induced persistence. Here we show that, in support of and consistent with our initial model, HipA-induced persistence depends not only on (p)ppGpp but also on the 10 mRNase-encoding TA modules, Lon protease, and polyphosphate. Importantly, observations with single cells convincingly show that the high level of (p)ppGpp caused by activation of HipA does not induce persistence in the absence of TA-encoded mRNases. Thus, slow growth per se does not induce persistence in the absence of TA-encoded toxins, placing these genes as central effectors of bacterial persistence.
Keywords: (p)ppGpp; HipA; bacterial persistence; single-cell analysis; toxin–antitoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bigger JW. Treatment of staphyloccal infections with penicillin by intermittent sterilisation. Lancet. 1944;244(6320):497–500.
-
- Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4(7):556–562. - PubMed
-
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

