Concise Review: Macrophages: Versatile Gatekeepers During Pancreatic β-Cell Development, Injury, and Regeneration
- PMID: 25848123
- PMCID: PMC4449100
- DOI: 10.5966/sctm.2014-0272
Concise Review: Macrophages: Versatile Gatekeepers During Pancreatic β-Cell Development, Injury, and Regeneration
Abstract
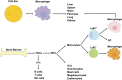
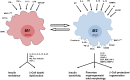
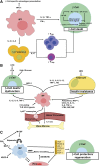
Macrophages are classically considered detrimental for pancreatic β-cell survival and function, thereby contributing to β-cell failure in both type 1 (T1D) and 2 (T2D) diabetes mellitus. In addition, adipose tissue macrophages negatively influence peripheral insulin signaling and promote obesity-induced insulin resistance in T2D. In contrast, recent data unexpectedly uncovered that macrophages are not only able to protect β cells during pancreatitis but also to orchestrate β-cell proliferation and regeneration after β-cell injury. Moreover, by altering their activation state, macrophages are able to improve insulin resistance in murine models of T2D. This review will elaborate on current insights in macrophage heterogeneity and on the evolving role of pancreas macrophages during organogenesis, tissue injury, and repair. Additional identification of macrophage subtypes and of their secreted factors might ultimately translate into novel therapeutic strategies for both T1D and T2D.
Significance: Diabetes mellitus is a pandemic disease, characterized by severe acute and chronic complications. Macrophages have long been considered prime suspects in the pathogenesis of both type 1 and 2 diabetes mellitus. In this concise review, current insights in macrophage heterogeneity and on the, as yet, underappreciated role of alternatively activated macrophages in insulin sensing and β-cell development/repair are reported. Further identification of macrophage subtypes and of their secreted factors might ultimately translate into novel therapeutic strategies for diabetes mellitus.
Keywords: Development; Injury; Macrophage; Pancreas; Regeneration; β Cell.
©AlphaMed Press.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

