Quantitative analysis of total β-subunit of human chorionic gonadotropin concentration in urine by immunomagnetic reduction to assist in the diagnosis of ectopic pregnancy
- PMID: 25848265
- PMCID: PMC4386805
- DOI: 10.2147/IJN.S81201
Quantitative analysis of total β-subunit of human chorionic gonadotropin concentration in urine by immunomagnetic reduction to assist in the diagnosis of ectopic pregnancy
Abstract
Background: The initial diagnosis of ectopic pregnancy depends on physical examination, ultrasound, and serial measurements of total β-subunit of human chorionic gonadotropin (hCGβ) concentrations in serum. The aim of this study was to explore the possibility of using quantitative analysis of total hCGβ in urine rather than in serum by immunomagnetic reduction (IMR) assay as an alternative method to diagnose an ectopic pregnancy.
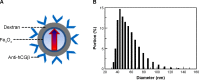
Methods: We established a standard calibration curve of IMR intensity against total hCGβ concentration based on standard hCGβ samples, and used an IMR assay to detect total hCGβ concentrations in the urine of pregnant women with lower abdominal pain and/or vaginal bleeding. The final diagnosis of ectopic pregnancy was based on ultrasound scans, operative findings, and pathology reports. In this prospective study, ten clinical samples were used to analyze the relationship of total hCGβ IMR signals between urine and serum. Furthermore, 20 clinical samples were used to analyze the relationship between urine IMR signals and serum levels of total hCGβ.
Results: The calibration curve extended from 0.01 ng/mL to 10,000 ng/mL with an excellent correlation (R(2)=0.999). In addition, an excellent correlation of total hCGβ IMR signals between urine and serum was noted (R(2)=0.994). Furthermore, a high correlation between urine IMR signals and serum levels of total hCGβ was noted (R(2)=0.862).
Conclusion: An IMR assay can quantitatively analyze total hCGβ concentrations in urine, and is a potential candidate for point-of-care testing to assist in the diagnosis of ectopic pregnancy.
Keywords: beta-subunit of human chorionic gonadotropin; ectopic pregnancy; immunomagnetic reduction; point-of-care.
Figures





References
-
- Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55:376–386. - PubMed
-
- Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72:1707–1714. - PubMed
-
- Kriebs JM, Fahey JO. Ectopic pregnancy. J Midwifery Womens Health. 2006;51:431–439. - PubMed
-
- Barnhart KT, Katz I, Hummel A, Gracia CR. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505–510. - PubMed
-
- Iles RK, Chard T. Molecular insights into the structure and function of human chorionic gonadotrophin. J Mol Endocrinol. 1993;10:217–234. - PubMed

