A Canadian national expert consensus on neoadjuvant therapy for breast cancer: linking practice to evidence and beyond
- PMID: 25848338
- PMCID: PMC4381790
- DOI: 10.3747/co.22.2328
A Canadian national expert consensus on neoadjuvant therapy for breast cancer: linking practice to evidence and beyond
Abstract
Background: Use of the neoadjuvant approach to treat breast cancer patients has increased since the early 2000s, but the overall pathway of care for such patients can be highly variable. The aim of our project was to establish a multidisciplinary consensus among clinicians with expertise in neoadjuvant therapy (nat) for breast cancer and to determine if that consensus reflects published methods used in randomized controlled trials (rcts) in this area.
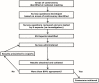
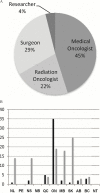
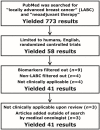
Methods: A modified Delphi protocol, which used iterative surveys administered to 85 experts across Canada, was established to obtain expert consensus concerning all aspects of the care pathway for patients undergoing nat for breast cancer. All rcts published between January 1, 1967, and December 1, 2012, were systematically reviewed. Data extracted from the rcts were analyzed to determine if the methods used matched the expert consensus for specific areas of nat management. A scoring system determined the strength of the agreement between the literature and the expert consensus.
Results: Consensus was achieved for all areas of the pathway of care for patients undergoing nat for breast cancer, with the exception of the role of magnetic resonance imaging in the pre-treatment or preoperative setting. The levels of agreement between the consensus statements and the published rcts varied, primarily because specific aspects of the pathway of care were not well described in the reviewed literature.
Conclusions: A true consensus of expert opinion concerning the pathway of care appropriate for patients receiving nat for breast cancer has been achieved. A review of the literature illuminated gaps in the evidence about some elements of nat management. Where evidence is available, agreement with expert opinion is strong overall. Our study is unique in its approach to establishing consensus among medical experts in this field and has established a pathway of care that can be applied in practice for patients receiving nat.
Keywords: Breast cancer; consensus; locally advanced breast cancer; neoadjuvant chemotherapy; neoadjuvant endocrine therapy; pathway of care.
Figures
References
-
- Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with her2-positive, clinical stage i–iii breast cancer (hannah study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–78. doi: 10.1016/S1470-2045(12)70329-7. - DOI - PubMed
-
- Untch M, Loibl S, Bischoff J, et al. on behalf of the German Breast Group (gbg) Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Group. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline–taxane-based chemotherapy (GeparQuinto, gbg 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–44. doi: 10.1016/S1470-2045(11)70397-7. - DOI - PubMed
-
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early her2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

