Scalable Architecture for Federated Translational Inquiries Network (SAFTINet) Technology Infrastructure for a Distributed Data Network
- PMID: 25848567
- PMCID: PMC4371513
- DOI: 10.13063/2327-9214.1027
Scalable Architecture for Federated Translational Inquiries Network (SAFTINet) Technology Infrastructure for a Distributed Data Network
Abstract
Introduction: Distributed Data Networks (DDNs) offer infrastructure solutions for sharing electronic health data from across disparate data sources to support comparative effectiveness research. Data sharing mechanisms must address technical and governance concerns stemming from network security and data disclosure laws and best practices, such as HIPAA.
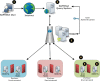
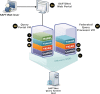
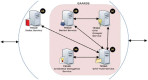
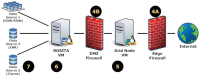
Methods: The Scalable Architecture for Federated Translational Inquiries Network (SAFTINet) deploys TRIAD grid technology, a common data model, detailed technical documentation, and custom software for data harmonization to facilitate data sharing in collaboration with stakeholders in the care of safety net populations. Data sharing partners host TRIAD grid nodes containing harmonized clinical data within their internal or hosted network environments. Authorized users can use a central web-based query system to request analytic data sets.
Discussion: SAFTINet DDN infrastructure achieved a number of data sharing objectives, including scalable and sustainable systems for ensuring harmonized data structures and terminologies and secure distributed queries. Initial implementation challenges were resolved through iterative discussions, development and implementation of technical documentation, governance, and technology solutions.
Keywords: Comparative Effectiveness; Data Use and Quality; De-identification; Health Information Technology; Research Networks; SAFTINet.
Figures
References
-
- Larsson ME, Kreuter M, Nordholm L. Is patient responsibility for managing musculoskeletal disorders related to self-reported better outcome of physiotherapy treatment? Physiotherapy theory and practice. 2010;26(5):308–17. Epub 2010/06/19. - PubMed
-
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA : the journal of the American Medical Association. 2003;290(12):1624–32. Epub 2003/09/25. - PubMed
-
- Slutsky JR, Clancy CM. Patient-centered comparative effectiveness research: essential for high-quality care. Arch Intern Med. 2010;170(5):403–4. Epub 2010/03/10. - PubMed
-
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. The New England journal of medicine. 2000;342(25):1878–86. Epub 2000/06/22. - PubMed
-
- Peddicord D, Waldo AB, Boutin M, Grande T, Gutierrez L., Jr A proposal to protect privacy of health information while accelerating comparative effectiveness research. Health affairs. 2010;29(11):2082–90. Epub 2010/11/03. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources