Accelerating regulatory progress in multi-institutional research
- PMID: 25848593
- PMCID: PMC4371517
- DOI: 10.13063/2327-9214.1076
Accelerating regulatory progress in multi-institutional research
Abstract
Purpose: Multi-institutional collaborations are necessary in order to create large and robust data sets that are needed to answer important comparative effectiveness research (CER) questions. Before scientific work can begin, a complex maze of administrative and regulatory requirements must be efficiently navigated to avoid project delays.
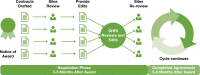
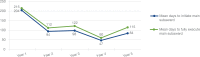
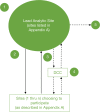
Innovation: Staff from research, regulatory, and administrative teams involved in three HMO Research Network (HMORN) multi-institutional collaborations developed and employed novel approaches: to secure and maintain Institutional Review Board (IRB) approvals; to enable data sharing, and to expedite subawards for two data-only minimal risk studies. These novel approaches accelerated required processes and approvals while maintaining regulatory, human subjects, and institutional protections.
Credibility: Outcomes from the processes described here are compared with processes outlined in the research and regulatory literature and with processes that have been used in previous multisite research collaborations.
Conclusion and discussion: Research, regulatory, and administrative staff are essential contributors to the success of multi-institutional collaborations. Their flexibility, creativity, and effective communication skills can lead to the development of efficient approaches to achieving the necessary oversight for these complex projects. Elements of these specific strategies can be adapted and used by other research networks. Other efforts in these areas should be evaluated and shared. The processes that help develop a "learning research system" play an important and complementary role in sustaining multi-institutional research collaborations.
Keywords: Administrative Efficiency; Data agreement; Institutional Review Board; Subaward; Subcontract.
Figures
References
-
- Maro JC, Platt R, Holmes JH, et al. Design of a national distributed health data network. Ann Intern Med. 2009;151:341–344. PM:19638403. - PubMed
-
- Toh S, Gagne JJ, Rassen JA, Fireman BH, Kulldorff M, Brown JS. Confounding adjustment in comparative effectiveness research conducted within distributed research networks. Med Care. 2013;51:S4–10. PM:23752258. - PubMed
-
- McWilliams R, Hoover-Fong J, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA. 2003;290:360–366. PM:12865377. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources