ATP4a is required for development and function of the Xenopus mucociliary epidermis - a potential model to study proton pump inhibitor-associated pneumonia
- PMID: 25848696
- PMCID: PMC4592800
- DOI: 10.1016/j.ydbio.2015.03.013
ATP4a is required for development and function of the Xenopus mucociliary epidermis - a potential model to study proton pump inhibitor-associated pneumonia
Abstract
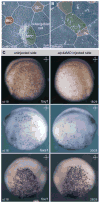
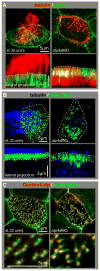
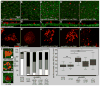
Proton pump inhibitors (PPIs), which target gastric H(+)/K(+)ATPase (ATP4), are among the most commonly prescribed drugs. PPIs are used to treat ulcers and as a preventative measure against gastroesophageal reflux disease in hospitalized patients. PPI treatment correlates with an increased risk for airway infections, i.e. community- and hospital-acquired pneumonia. The cause for this correlation, however, remains elusive. The Xenopus embryonic epidermis is increasingly being used as a model to study airway-like mucociliary epithelia. Here we use this model to address how ATP4 inhibition may affect epithelial function in human airways. We demonstrate that atp4a knockdown interfered with the generation of cilia-driven extracellular fluid flow. ATP4a and canonical Wnt signaling were required in the epidermis for expression of foxj1, a transcriptional regulator of motile ciliogenesis. The ATP4/Wnt module activated foxj1 downstream of ciliated cell fate specification. In multiciliated cells (MCCs) of the epidermis, ATP4a was also necessary for normal myb expression, apical actin formation, basal body docking and alignment of basal bodies. Furthermore, ATP4-dependent Wnt/β-catenin signaling in the epidermis was a prerequisite for foxa1-mediated specification of small secretory cells (SSCs). SSCs release serotonin and other substances into the medium, and thereby regulate ciliary beating in MCCs and protect the epithelium against infection. Pharmacological inhibition of ATP4 in the mature mucociliary epithelium also caused a loss of MCCs and led to impaired mucociliary clearance. These data strongly suggest that PPI-associated pneumonia in human patients might, at least in part, be linked to dysfunction of mucociliary epithelia of the airways.
Keywords: ATP4; Cilia; Proton pump inhibitor; Small secretory cells; Wnt; Xenopus laevis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
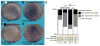


Similar articles
-
ATP4 and ciliation in the neuroectoderm and endoderm of Xenopus embryos and tadpoles.Data Brief. 2015 Apr 20;4:22-31. doi: 10.1016/j.dib.2015.04.003. eCollection 2015 Sep. Data Brief. 2015. PMID: 26217756 Free PMC article.
-
ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development.Cell Rep. 2012 May 31;1(5):516-27. doi: 10.1016/j.celrep.2012.03.005. Epub 2012 Apr 20. Cell Rep. 2012. PMID: 22832275
-
A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles.Development. 2014 Apr;141(7):1526-33. doi: 10.1242/dev.102343. Epub 2014 Mar 5. Development. 2014. PMID: 24598162
-
Xenopus epidermal and endodermal epithelia as models for mucociliary epithelial evolution, disease, and metaplasia.Genesis. 2021 Feb;59(1-2):e23406. doi: 10.1002/dvg.23406. Epub 2021 Jan 5. Genesis. 2021. PMID: 33400364 Review.
-
Building a ciliated epithelium: Transcriptional regulation and radial intercalation of multiciliated cells.Curr Top Dev Biol. 2021;145:3-39. doi: 10.1016/bs.ctdb.2020.08.001. Epub 2020 Sep 14. Curr Top Dev Biol. 2021. PMID: 34074533 Review.
Cited by
-
Distinct Spatiotemporally Dynamic Wnt-Secreting Niches Regulate Proximal Airway Regeneration and Aging.Cell Stem Cell. 2020 Sep 3;27(3):413-429.e4. doi: 10.1016/j.stem.2020.06.019. Epub 2020 Jul 27. Cell Stem Cell. 2020. PMID: 32721381 Free PMC article.
-
What we can learn from a tadpole about ciliopathies and airway diseases: Using systems biology in Xenopus to study cilia and mucociliary epithelia.Genesis. 2017 Jan;55(1-2):10.1002/dvg.23001. doi: 10.1002/dvg.23001. Genesis. 2017. PMID: 28095645 Free PMC article. Review.
-
Mucociliary Wnt signaling promotes cilia biogenesis and beating.Nat Commun. 2023 Mar 6;14(1):1259. doi: 10.1038/s41467-023-36743-2. Nat Commun. 2023. PMID: 36878953 Free PMC article.
-
ΔN-Tp63 Mediates Wnt/β-Catenin-Induced Inhibition of Differentiation in Basal Stem Cells of Mucociliary Epithelia.Cell Rep. 2019 Sep 24;28(13):3338-3352.e6. doi: 10.1016/j.celrep.2019.08.063. Cell Rep. 2019. PMID: 31553905 Free PMC article.
-
Xenbase: Facilitating the Use of Xenopus to Model Human Disease.Front Physiol. 2019 Feb 26;10:154. doi: 10.3389/fphys.2019.00154. eCollection 2019. Front Physiol. 2019. PMID: 30863320 Free PMC article. Review.
References
-
- Altman KW, Waltonen JD, Tarjan G, Radosevich Ja, Haines GK. Human lung mucous glands manifest evidence of the H+/K+-ATPase proton pump. Ann Otol Rhinol Laryngol. 2007;116:229–34. - PubMed
-
- Beyer T, Danilchik M, Thumberger T, Vick P, Tisler M, Schneider I, Bogusch S, Andre P, Ulmer B, Walentek P, Niesler B, Blum M, Schweickert A. Serotonin Signaling Is Required for Wnt-Dependent GRP Specification and Leftward Flow in Xenopus. Curr Biol. 2012;22:1–7. doi: 10.1016/j.cub.2011.11.027. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

